Press release
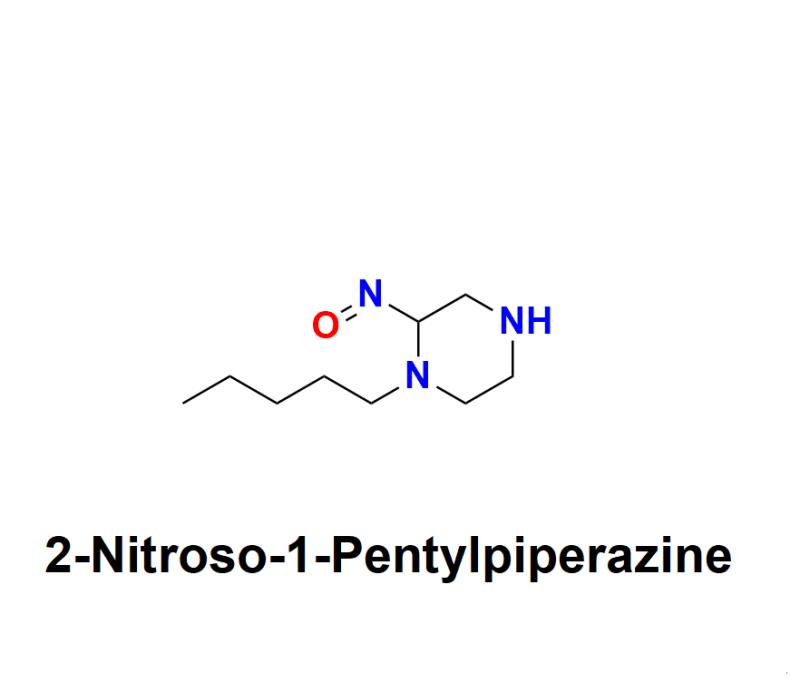
2-Nitroso-1-Pentylpiperazine Impurities in Pharmaceutical Manufacturing: Exploring the Risks, Sources, Health Hazards, and Regulatory Compliance Challenges, with Expert Solutions from Aquigen Bio Sciences for Industry Leaders
2-Nitroso-1-Pentylpiperazine has recently emerged as a significant area of concern within the pharmaceutical industry. As a potentially carcinogenic impurity, this nitroso compound poses substantial risks if present in medications, necessitating urgent attention from pharmaceutical companies worldwide. Regulatory authorities are increasingly focused on this compound due to the health risks it poses, leading to tighter guidelines and heightened safety measures to protect consumers. This press release highlights the health hazards associated with 2-Nitroso-1-Pentylpiperazine, its origins, and why the pharmaceutical industry must prioritize its control.Learn more about 2-Nitroso-1-Pentylpiperazine: https://aquigenbio.com/product/2-nitroso-1-pentylpiperazine/
2-Nitroso-1-Pentylpiperazine is one of the nitroso compounds, a category of chemicals known to be highly reactive and toxic, with many classified as probable human carcinogens. These impurities can emerge during the drug manufacturing process as by-products, often through nitrosation reactions involving nitrites and other reactive agents. The potential for these impurities to affect human health has brought a new level of scrutiny, underscoring the urgent need for the pharmaceutical sector to adopt proactive impurity detection and management strategies.
Health Hazards Posed By 2-Nitroso-1-Pentylpiperazine:
Studies and recent data indicate that 2-Nitroso-1-Pentylpiperazine poses notable health hazards if it contaminates pharmaceutical products. Even at low levels, the compound's structure allows it to interact with human DNA, potentially leading to mutations that elevate cancer risk. This particular nitroso impurity, due to its stability and reactivity, may disrupt cellular processes and impair vital functions, contributing to the formation of tumors in organs such as the liver, kidneys, and gastrointestinal tract. Other reported health risks associated with nitroso impurities include long-term toxicity and organ damage, underscoring the need for careful management of 2-Nitroso-1-Pentylpiperazine levels in medications.
The severity of these health risks has led regulatory bodies such as the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) to enforce stringent limits on nitroso impurities, with specific thresholds outlined for pharmaceutical products. The challenge for pharmaceutical manufacturers lies in not only detecting 2-Nitroso-1-Pentylpiperazine with high accuracy but also eliminating or controlling its presence through refined manufacturing protocols.
Understanding the Sources of 2-Nitroso-1-Pentylpiperazine:
In the context of pharmaceutical production, nitroso impurities like 2-Nitroso-1-Pentylpiperazine can arise from a variety of sources. The most common pathways include interactions with nitrites in acidic environments and contamination during synthesis. While these impurities are usually formed inadvertently, their presence in medications can often be traced back to reactions involving amines, amides, and other reactive groups under certain conditions.
Additionally, raw materials used in drug synthesis may contain trace amounts of nitrites, which under specific conditions can lead to the formation of nitroso impurities. This emphasizes the importance of using high-quality raw materials, as well as performing regular monitoring and testing to ensure impurity levels remain within permissible limits. Proper storage and controlled production environments can also help minimize the risks associated with 2-Nitroso-1-Pentylpiperazine formation, making these considerations a critical part of quality control in pharmaceutical manufacturing.
Get in Touch with Us - https://aquigenbio.com/contact-us/
Strengthening Regulatory Compliance and Consumer Safety
The presence of 2-Nitroso-1-Pentylpiperazine has implications not only for pharmaceutical safety but also for a company's reputation, financial stability, and regulatory standing. Regulatory bodies have set rigorous standards for nitroso impurities, with severe consequences for non-compliance, including product recalls, financial penalties, and potential legal actions. For pharmaceutical companies, adhering to these standards has become a crucial step in securing market access and maintaining consumer trust.
To address these challenges, companies are increasingly turning to risk management frameworks that identify critical points where nitroso impurities might arise during production. Such frameworks emphasize process optimization and the implementation of best practices to minimize nitroso compound formation. Preventive strategies, including alternative synthetic pathways, proper storage conditions, and supplier evaluations, are essential to reducing the chances of contamination from 2-Nitroso-1-Pentylpiperazine.
Conclusion:
As the risks associated with 2-Nitroso-1-Pentylpiperazine continue to garner attention, pharmaceutical manufacturers are urged to strengthen their quality control and impurity testing practices.
Aquigen Bio Sciences stands as the premier resource for pharmaceutical companies seeking to detect, analyze, and control 2-Nitroso-1-Pentylpiperazine. With a specialized focus on impurity standards, Aquigen Bio Sciences is equipped with state-of-the-art analytical technology and a dedicated team of experts to support rigorous impurity management at every stage of production. The company's comprehensive solutions range from advanced testing capabilities to precise impurity profiling and customized consultancy services tailored to meet the unique needs of each client. This proactive approach helps pharmaceutical manufacturers not only meet but exceed regulatory requirements, ensuring consumer safety and product integrity.
For manufacturers navigating the complexities of nitroso impurities, Aquigen Bio Sciences offers trusted support and expertise, making it an invaluable partner in maintaining high standards of pharmaceutical safety and compliance. Through its dedication and expertise, Aquigen Bio Sciences helps secure the integrity of pharmaceuticals, protecting both the industry and the consumers it serves. To learn more about 2-Nitroso-1-Pentylpiperazine Nitroso Impurity Standards, connect with the Aquigen Bio Sciences team today.
Similar Trending Products:
1) N-Nitroso Chlorobenzhydryl Piperazine: https://aquigenbio.com/product/n-nitroso-chlorobenzhydryl-piperazine/
2) N-Nitroso Enalapril: https://aquigenbio.com/product/n-nitroso-enalapril-2/
3) N-Nitroso Zolpyridine: https://aquigenbio.com/product/n-nitroso-zolpyridine/
Contact Us:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen Bio Sciences:
Aquigen Bio Sciences is a renowned contract research organization based in Pune, India, specializing in impurity standards and comprehensive solutions for the pharmaceutical industry. With a focus on advancing pharmaceutical safety and compliance, Aquigen provides expert guidance on identifying, quantifying, and managing impurities in various drug formulations. Their commitment to quality and regulatory excellence positions them as a trusted partner for manufacturers striving to meet stringent safety standards and improve patient outcomes.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release 2-Nitroso-1-Pentylpiperazine Impurities in Pharmaceutical Manufacturing: Exploring the Risks, Sources, Health Hazards, and Regulatory Compliance Challenges, with Expert Solutions from Aquigen Bio Sciences for Industry Leaders here
News-ID: 3735651 • Views: …
More Releases from Aquigen Biosciences
Precision Standards for Oncology Research: Exploring Abemaciclib Impurity 1 and …
In the ever-evolving field of targeted cancer therapy, Abemaciclib has emerged as a pivotal agent in the treatment of hormone receptor-positive (HR+), HER2-negative advanced or metastatic breast cancer. As researchers and pharmaceutical developers continue to innovate in oncology, the importance of impurity profiling and the availability of reliable Abemaciclib impurity standards has never been greater.
At the forefront of pharmaceutical impurity standards, Aquigen Bio is proud to support global manufacturers, CROs,…
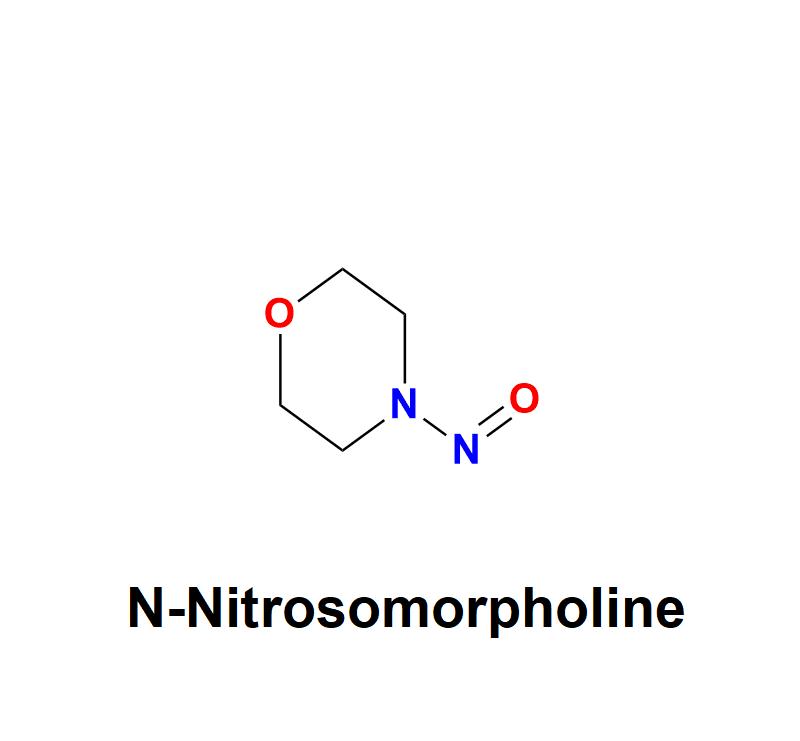
N-Nitrosomorpholine: Addressing Pharmaceutical Safety Challenges with Aquigen Bi …
N-Nitrosomorpholine, a compound belonging to the nitrosamine family, has garnered significant attention in the pharmaceutical and healthcare industries due to its potential carcinogenic risks. This chemical impurity, often found as a byproduct in manufacturing processes, poses serious challenges to drug safety and human health, necessitating stringent monitoring and control measures from pharmaceutical companies.
Learn more about N-Nitrosomorpholine: https://aquigenbio.com/product/n-nitrosomorpholine/
Understanding N-Nitrosomorpholine:
N-Nitrosomorpholine is a nitrosamine impurity characterized by its chemical structure, which includes…
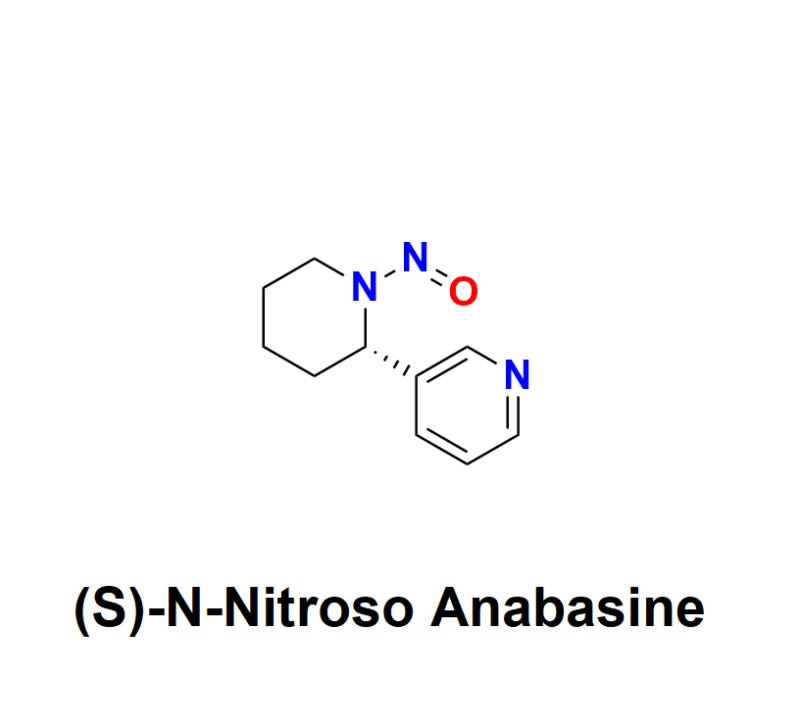
(S)-N-Nitroso Anabasine: Understanding the Risks, Regulatory Challenges, and How …
(S)-N-Nitroso Anabasine, a nitrosamine impurity, has raised significant safety concerns within the pharmaceutical industry. Recognized as a probable human carcinogen, this impurity has become a focal point for global regulatory agencies and manufacturers alike, urging a renewed emphasis on detection, prevention, and management.
Learn more about (S)-N-Nitroso Anabasine: https://aquigenbio.com/product/s-n-nitroso-anabasine/
What Is (S)-N-Nitroso Anabasine?
(S)-N-Nitroso Anabasine belongs to the family of nitrosamines, compounds formed through a chemical reaction known as nitrosation. This…
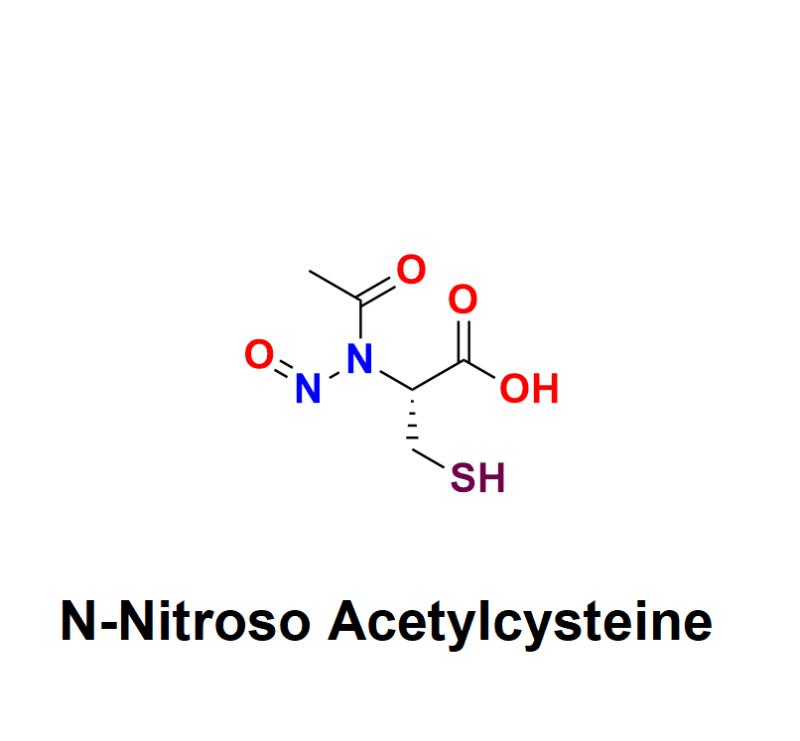
N-Nitroso Acetylcysteine: A Critical Concern in Pharmaceuticals - Exploring Haza …
N-Nitroso Acetylcysteine has emerged as a critical topic of concern in the pharmaceutical industry. As a member of the nitrosamine family, it is a potential impurity that poses significant health risks, including carcinogenicity, even in trace amounts. With increasing regulatory scrutiny on nitrosamine impurities, pharmaceutical manufacturers must address the presence of compounds like N-Nitroso Acetylcysteine to protect public health and ensure compliance with global standards.
Learn more about N-Nitroso Acetylcysteine: https://aquigenbio.com/product/n-nitroso-acetylcysteine/…
More Releases for Nitroso
Riociguat N-Nitroso Des Formyl Impurity - Reliable Reference Standard for Advanc …
Riociguat N-Nitroso Des Formyl Impurity is a premium-quality analytical reference material developed to support research, testing, and quality control in the pharmaceutical industry.
As regulatory guidelines tighten worldwide, accurate detection, quantification, and control of nitroso impurities have become an essential part of drug development and manufacturing.
This compound plays a vital role in ensuring that formulations containing Riociguat meet the highest safety and compliance standards.
The Growing Importance of Nitroso Impurity…
Tizanidine Nitroso Impurity 1 - High-Purity Reference Standard for Reliable Impu …
In the highly regulated world of pharmaceutical manufacturing, impurity profiling plays a critical role in ensuring drug safety, efficacy, and regulatory compliance. Among the wide range of impurity reference standards required during API development and validation, Tizanidine Nitroso Impurity 1 stands out as a vital compound for researchers and manufacturers working on Tizanidine-based formulations.
At Aquigen Bio, we are proud to offer Tizanidine Nitroso Impurity 1, a high-purity reference standard meticulously…
Aquigen Bio Unveils N-Nitroso Felodipine: A New Benchmark in Pharmaceutical Refe …
In an era where drug safety and efficacy are paramount, the pharmaceutical industry faces increasing pressure to develop robust analytical methods that ensure the purity and quality of medicinal products. N-Nitroso Felodipine is specifically engineered to address these challenges. It serves as an indispensable tool for Analytical Method Development (AMD) and Analytical Method Validation (AMV), providing a reliable benchmark against which new and existing analytical procedures can be assessed for…
N-Nitroso Impurities: Carcinogenic Risks, Analytical Challenges, and Compliance …
N-Nitroso impurities are emerging as a significant concern in the pharmaceutical industry due to their potential carcinogenicity. These compounds, formed during the manufacturing or storage of drug products, have raised alarms among regulatory agencies across the globe. Pharmaceutical companies face mounting pressure to identify, analyze, and mitigate these impurities through stringent guidelines to ensure patient safety.
The serious risks posed by N-Nitroso compounds stem from their ability to induce genetic mutations…
Bumetanide Nitroso Impurity: Mitigating Compliance Risks and Integrating Innovat …
Bumetanide Nitroso Impurity has emerged as a significant concern in the pharmaceutical industry, particularly for companies involved in the development and manufacturing of medications used to manage edema. As awareness grows about the potential risks associated with nitroso impurities, regulatory agencies worldwide are increasing scrutiny over pharmaceutical products. This press release aims to address the challenges posed by Bumetanide Nitroso Impurity, outline the regulatory landscape, and highlight innovative practices that…
Sumatriptan Nitroso Impurity: Navigating Compliance Challenges and Implementing …
Sumatriptan Nitroso Impurity has become a pressing concern in the pharmaceutical industry, as companies grapple with significant compliance challenges related to these potentially harmful contaminants. As Sumatriptan, a widely prescribed medication for migraine relief, comes under increasing scrutiny from regulatory bodies, pharmaceutical companies must navigate a complex landscape to ensure product safety and adhere to evolving regulatory standards.
What is the issue?
Nitroso impurities have emerged as a pressing concern within the…