Press release
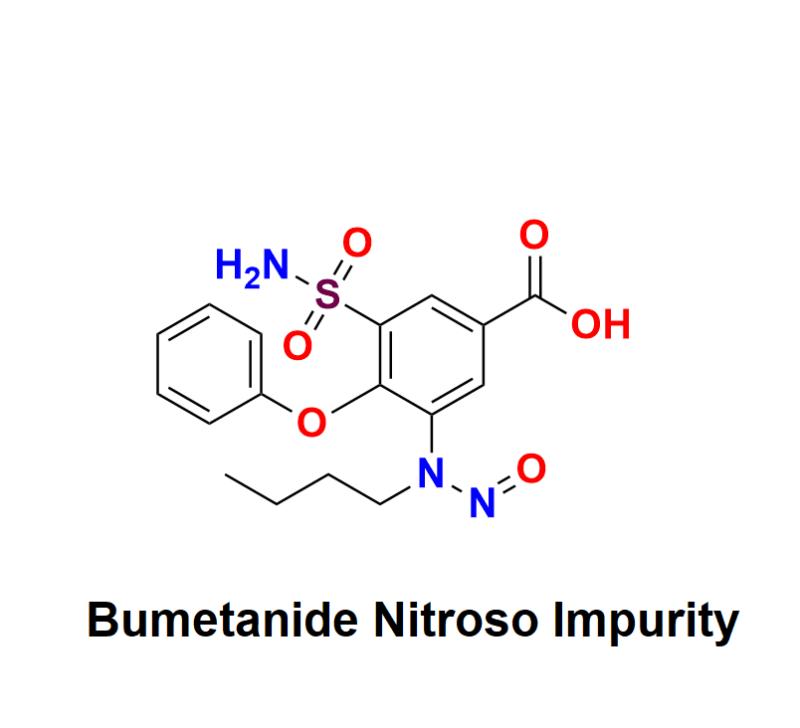
Bumetanide Nitroso Impurity: Mitigating Compliance Risks and Integrating Innovative Practices to Uphold Safety and Regulatory Standards for Pharmaceutical Companies in the Complex Landscape of Nitroso Contaminants in Edema Management
Bumetanide Nitroso Impurity has emerged as a significant concern in the pharmaceutical industry, particularly for companies involved in the development and manufacturing of medications used to manage edema. As awareness grows about the potential risks associated with nitroso impurities, regulatory agencies worldwide are increasing scrutiny over pharmaceutical products. This press release aims to address the challenges posed by Bumetanide Nitroso Impurity, outline the regulatory landscape, and highlight innovative practices that pharmaceutical companies can adopt to mitigate compliance risks while ensuring patient safety.Understanding Bumetanide and Its Importance in Edema Management:
Bumetanide is a loop diuretic commonly prescribed to treat edema associated with heart failure, liver cirrhosis, and renal disorders. Its effectiveness in promoting the excretion of excess fluid makes it a vital component of treatment protocols for patients suffering from these conditions. However, the increasing awareness of nitroso impurities in pharmaceuticals poses significant risks not only to patient safety but also to the compliance landscape of pharmaceutical manufacturers.
Learn more about Bumetanide Nitroso Impurity: https://aquigenbio.com/product/bumetanide-nitroso-impurity/
The Challenge of Bumetanide Nitroso Impurities:
Nitroso impurities are classified as potential carcinogens and can form during the synthesis or storage of pharmaceutical products. They present unique challenges for manufacturers as they are difficult to detect and quantify, leading to potential safety concerns and regulatory compliance issues. The emergence of nitroso impurities, including Bumetanide Nitroso Impurity, underscores the need for a comprehensive approach to risk management within the pharmaceutical industry.
The presence of nitroso impurities in Bumetanide and other medications raises significant safety concerns for patients. As regulatory bodies become increasingly vigilant, pharmaceutical companies face heightened scrutiny and potential repercussions, including product recalls, regulatory actions, and damage to their reputations.
Regulatory Landscape: Navigating Compliance Challenges:
The regulatory environment concerning Bumetanide Nitroso Impurity has experienced significant changes in recent years, driven by heightened awareness of the risks associated with nitroso contaminants. Regulatory bodies such as the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency) have issued comprehensive guidance aimed at aiding pharmaceutical manufacturers in the identification, assessment, and management of these impurities. These guidelines underscore the importance of establishing robust testing protocols specifically for Bumetanide, conducting thorough risk assessments, and ensuring meticulous documentation to maintain compliance with stringent safety standards.
Pharmaceutical companies must remain vigilant in their compliance efforts to mitigate the risks associated with nitroso impurities. Failure to adequately address these concerns can result in serious consequences, including regulatory penalties, financial losses, and diminished public trust in the safety of their products.
Get in Touch with Us - https://aquigenbio.com/contact-us/
Mitigating Compliance Risks: Innovative Practices for Pharmaceutical Companies:
To navigate the complexities associated with Bumetanide Nitroso Impurity and other nitroso contaminants, pharmaceutical companies can implement a range of innovative practices aimed at ensuring compliance and upholding safety standards.
These practices may include:
1. Enhanced Analytical Testing: Adopting advanced analytical methodologies, such as liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry (GC-MS), enables manufacturers to detect and quantify nitroso impurities at trace levels. Investing in state-of-the-art analytical technology can help companies identify potential risks early in the development process.
2. Comprehensive Risk Assessments: Conducting thorough risk assessments is essential for identifying potential sources of nitroso impurity formation. By evaluating raw materials, manufacturing processes, and storage conditions, companies can proactively address risks and develop effective control strategies.
3. Cross-Department Collaboration: Fostering collaboration between various departments, including quality assurance, regulatory affairs, and research and development, is crucial for establishing a comprehensive compliance framework. By encouraging communication and knowledge sharing, pharmaceutical companies can better align their practices with regulatory expectations.
4. Continuous Monitoring Protocols: Implementing continuous monitoring and testing protocols throughout the manufacturing process can help identify nitroso impurities before they reach the final product stage. This includes routine testing of raw materials, intermediates, and finished products to ensure compliance with established safety standards.
5. Supplier Quality Management: Ensuring the quality of raw materials and active pharmaceutical ingredients (APIs) is vital in preventing nitroso impurities. Establishing stringent supplier quality management systems and conducting regular audits can help mitigate risks associated with raw material contamination.
Upholding Safety Standards: Protecting Patients and Maintaining Trust
Patient safety remains a top priority for pharmaceutical companies, and the presence of nitroso impurities raises serious concerns. To protect patient health and uphold public trust, manufacturers must take proactive measures to mitigate risks associated with Bumetanide Nitroso Impurity and other nitroso contaminants. By integrating innovative practices and fostering a culture of compliance, companies can demonstrate their commitment to patient safety while enhancing the overall quality of their products.
Conclusion:
As the pharmaceutical industry continues to navigate the complex landscape of nitroso contaminants, addressing compliance risks associated with Bumetanide Nitroso Impurity is essential. By integrating innovative practices, conducting thorough risk assessments, and fostering collaboration across departments, companies can uphold safety and regulatory standards while maintaining patient trust. A commitment to proactive compliance strategies will not only protect public health but also contribute to the long-term success and reputation of pharmaceutical organizations in a competitive market.
Similar Trending Products:
1) Abacavir Nitroso Impurity - https://aquigenbio.com/product/abacavir-nitroso-impurity/
2) 1,4-Dinitrosopiperazine - https://aquigenbio.com/product/14-dinitrosopiperazine/
3) Fostamatinib Nitroso Impurity 1 - https://aquigenbio.com/product/fostamatinib-nitroso-impurity-1/
Contact Us:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen Bio Sciences:
Aquigen Bio Sciences is a premier contract research organization located in Pune, India, dedicated to providing cutting-edge solutions for impurity standards and regulatory compliance in the pharmaceutical industry. Specializing in the analysis and management of nitroso impurities, including Bumetanide Nitroso Impurity, the company is committed to enhancing safety and quality in drug development. By partnering with pharmaceutical companies, Aquigen Bio Sciences helps navigate the regulatory landscape, ensuring that patient care remains the top priority while addressing the complexities associated with nitroso contaminants.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Bumetanide Nitroso Impurity: Mitigating Compliance Risks and Integrating Innovative Practices to Uphold Safety and Regulatory Standards for Pharmaceutical Companies in the Complex Landscape of Nitroso Contaminants in Edema Management here
News-ID: 3697551 • Views: …
More Releases from Aquigen Biosciences
Precision Standards for Oncology Research: Exploring Abemaciclib Impurity 1 and …
In the ever-evolving field of targeted cancer therapy, Abemaciclib has emerged as a pivotal agent in the treatment of hormone receptor-positive (HR+), HER2-negative advanced or metastatic breast cancer. As researchers and pharmaceutical developers continue to innovate in oncology, the importance of impurity profiling and the availability of reliable Abemaciclib impurity standards has never been greater.
At the forefront of pharmaceutical impurity standards, Aquigen Bio is proud to support global manufacturers, CROs,…
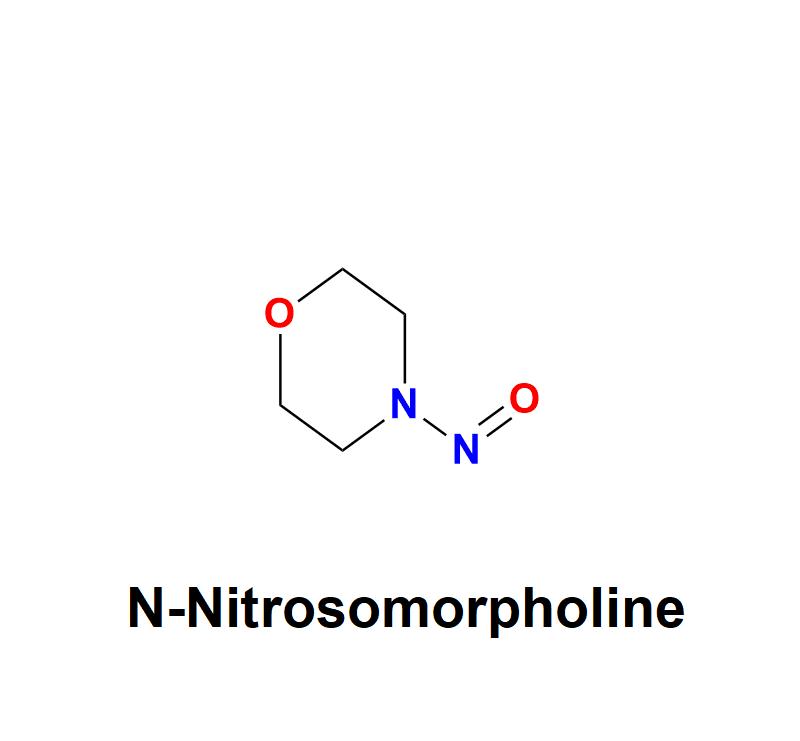
N-Nitrosomorpholine: Addressing Pharmaceutical Safety Challenges with Aquigen Bi …
N-Nitrosomorpholine, a compound belonging to the nitrosamine family, has garnered significant attention in the pharmaceutical and healthcare industries due to its potential carcinogenic risks. This chemical impurity, often found as a byproduct in manufacturing processes, poses serious challenges to drug safety and human health, necessitating stringent monitoring and control measures from pharmaceutical companies.
Learn more about N-Nitrosomorpholine: https://aquigenbio.com/product/n-nitrosomorpholine/
Understanding N-Nitrosomorpholine:
N-Nitrosomorpholine is a nitrosamine impurity characterized by its chemical structure, which includes…
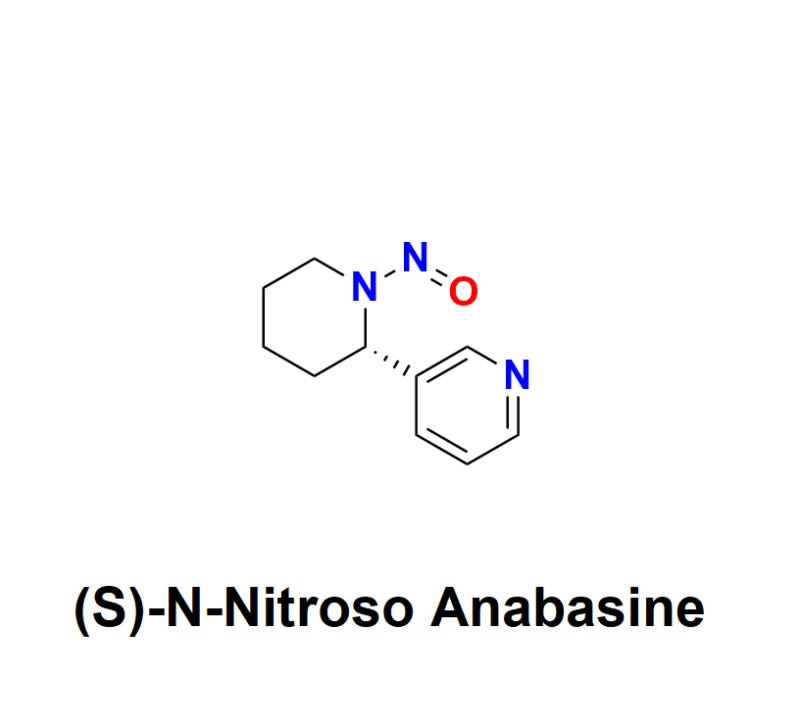
(S)-N-Nitroso Anabasine: Understanding the Risks, Regulatory Challenges, and How …
(S)-N-Nitroso Anabasine, a nitrosamine impurity, has raised significant safety concerns within the pharmaceutical industry. Recognized as a probable human carcinogen, this impurity has become a focal point for global regulatory agencies and manufacturers alike, urging a renewed emphasis on detection, prevention, and management.
Learn more about (S)-N-Nitroso Anabasine: https://aquigenbio.com/product/s-n-nitroso-anabasine/
What Is (S)-N-Nitroso Anabasine?
(S)-N-Nitroso Anabasine belongs to the family of nitrosamines, compounds formed through a chemical reaction known as nitrosation. This…
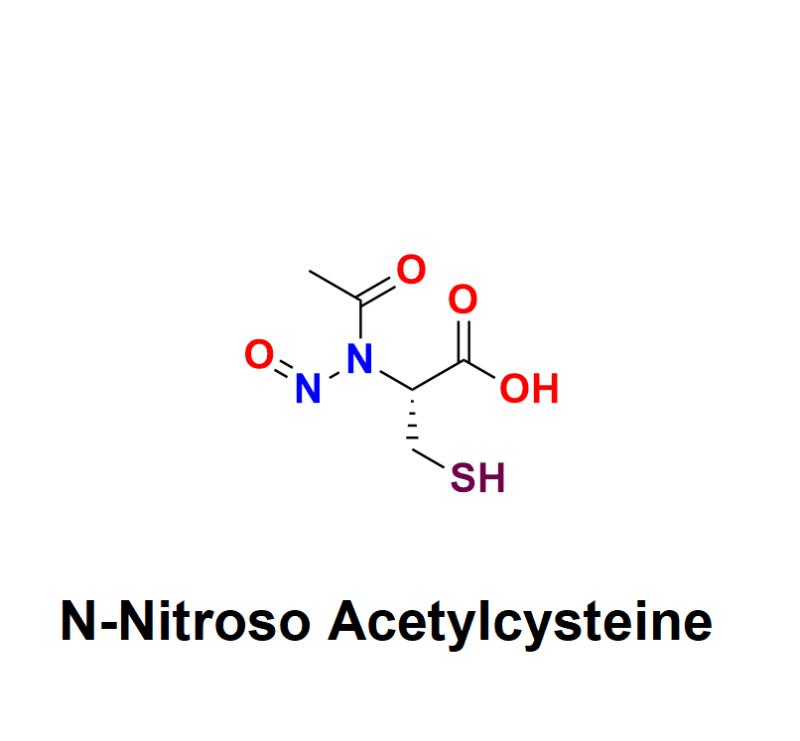
N-Nitroso Acetylcysteine: A Critical Concern in Pharmaceuticals - Exploring Haza …
N-Nitroso Acetylcysteine has emerged as a critical topic of concern in the pharmaceutical industry. As a member of the nitrosamine family, it is a potential impurity that poses significant health risks, including carcinogenicity, even in trace amounts. With increasing regulatory scrutiny on nitrosamine impurities, pharmaceutical manufacturers must address the presence of compounds like N-Nitroso Acetylcysteine to protect public health and ensure compliance with global standards.
Learn more about N-Nitroso Acetylcysteine: https://aquigenbio.com/product/n-nitroso-acetylcysteine/…
More Releases for Bumetanide
Congestive Heart Failure Treatment Landscape: FDA Approves Enbumyst, First Self- …
DelveInsight Business Research's analysis underscores the transformative implications of the FDA's approval of ENBUMYST (bumetanide nasal spray) by Corstasis Therapeutics Inc. (NASDAQ: CORT). This landmark decision delivers the first self-administered outpatient alternative to intravenous loop diuretics, promising enhanced convenience for the ~21 million adults living with congestive heart failure (CHF) in the 7MM.
Key Congestive Heart Failure Market Highlights
*
Enbumyst's approval is poised to be a Congestive Heart Failure market driver, expanding…
Global Diuretics Market Analysis 2025-2030: Growth Drivers, Challenges, And Oppo …
The Diuretics Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
How Big Is the Diuretics Market Size Expected to Be by 2034?
The diuretics market has grown steadily, increasing from $0.74 billion in 2024 to $0.77 billion in 2025 with a CAGR of 4.5%. Growth drivers…
Bumetanide Tablets Market | Valuates Reports
Bumetanide Tablets Market
Bumetanide is used to treat swelling caused by heart failure or liver or kidney disease.
The global Bumetanide Tablets market was valued at US$ million in 2022 and is anticipated to reach US$ million by 2029, witnessing a CAGR of % during the forecast period 2023-2029. The influence of COVID-19 and the Netherlands-Ukraine War were considered while estimating market sizes.
Download Free Sample: https://reports.valuates.com/request/sample/QYRE-Auto-4S7216/Global_Bumetanide_Tablets_Market_Insights_Forecast_to_2028
North American market for Bumetanide Tablets is…
Autism Spectrum Disorder Therapeutics Market Major Players F. Hoffmann-La Roche …
"Coherent Market Insights announced that it’s published an exclusive report namely Global Autism Spectrum Disorder Therapeutics Market by Manufacturers, Regions, Type, and Application, Forecast to 2027 in its research database with report summary, table of content, research methodologies, and data sources. The research study offers a substantial knowledge platform for entrants and investors as well as veteran companies, manufacturers functioning in the Worldwide Autism Spectrum Disorder Therapeutics Market. This is…
Autism Spectrum Disorder Therapeutics Market Experts Analysis To focuses on top …
The growing prevalence of ASD worldwide is fostering the growth of the autism spectrum disorder therapeutics market. According to the data published in Our World in Data in 2017, around 62 million people worldwide were estimated to have ASD in 2016. Additionally, under 50 million of these cases were found in males. Of this 62 million with ASD, around 18 million had only Autism and 44 million had Asperger syndrome…
Autism Spectrum Disorder Therapeutics Market Size was Valued at US$ 2,169.3 mill …
Autism Spectrum Disorder (ASD) is a neurodegenerative disorder, which is associated and often overlaps with other conditions such as epilepsy, frequent infections, sleep disorders, anxiety, depression, and gastrointestinal problems. ASD is only diagnosed by developmental and comprehensive tests. It can be detected at eighteen months to two years of age. Drugs such as Aripiprazole, Risperidone, and Melatonin are U.S. Food and Drug Administration (FDA) approved drugs that aid in the…