Press release
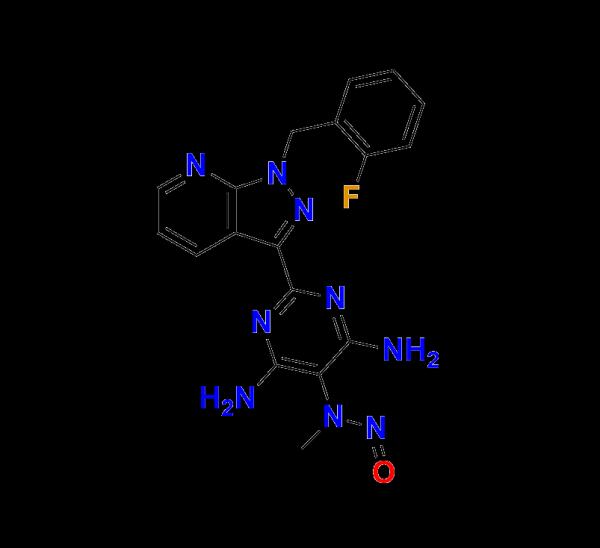
Riociguat N-Nitroso Des Formyl Impurity - Reliable Reference Standard for Advanced Pharmaceutical Analysis
Riociguat N-Nitroso Des Formyl Impurity is a premium-quality analytical reference material developed to support research, testing, and quality control in the pharmaceutical industry.As regulatory guidelines tighten worldwide, accurate detection, quantification, and control of nitroso impurities have become an essential part of drug development and manufacturing.
This compound plays a vital role in ensuring that formulations containing Riociguat meet the highest safety and compliance standards.
The Growing Importance of Nitroso Impurity Testing
Over the past few years, nitroso impurities have gained significant attention from health authorities such as the USFDA, EMA, and CDSCO. These compounds, belonging to the nitrosamine family, are known for their potential genotoxic risks, making their monitoring and control critical.
Riociguat, a soluble guanylate cyclase (sGC) stimulator used in the treatment of pulmonary hypertension, must be manufactured and stored in a way that minimizes the formation of nitroso impurities.
The presence of Riociguat N-Nitroso Des Formyl Impurity could indicate degradation pathways or contamination sources that require immediate attention.
For researchers, analytical laboratories, and pharmaceutical manufacturers, using a certified reference standard for this impurity enables accurate method development, validation, and batch release testing in compliance with ICH M7(R2) guidelines.
Explore : https://aquigenbio.com/product/riociguat-n-nitroso-des-formyl-impurity/
High-Purity Reference Standard for Confident Results
Aquigen Bio provides Riociguat N-Nitroso Des Formyl Impurity with exceptional purity and detailed characterization data to ensure confidence in results. Each batch is supported by a comprehensive Certificate of Analysis (CoA) containing:
HPLC purity data
Mass spectrometry (MS) results
NMR spectra
Storage and handling instructions
Our manufacturing process follows stringent quality protocols to ensure that the impurity profile is well-defined, allowing laboratories to rely on this standard for both qualitative and quantitative analysis.
Applications in Pharmaceutical Research & Quality Control
The use of Riociguat N-Nitroso Des Formyl Impurity spans several key areas:
1. Method Development & Validation
Analytical teams can use this impurity to develop sensitive and specific detection methods using techniques like LC-MS/MS, GC-MS, and HPLC-UV.
2. Stability Studies
Monitoring the formation of nitroso impurities under different environmental and storage conditions helps determine the stability profile of Riociguat formulations.
3. Regulatory Compliance
With nitrosamine limits now strictly regulated, having an authentic reference standard is essential for meeting guidelines and avoiding compliance risks.
4. Toxicological Risk Assessment
Accurate quantification supports toxicology studies, helping determine acceptable daily intake levels for patient safety.
Belongs to the Nitroso Impurity Standards Category
This product is part of our Nitroso Impurity Standards portfolio, which includes a wide range of certified nitrosamine and nitroso-related impurities for different APIs.
Aquigen Bio's nitroso impurity standards are developed to meet the needs of pharmaceutical manufacturers facing evolving regulatory requirements.
Our expertise in nitroso chemistry allows us to deliver reliable reference materials for high-precision analysis, minimizing the risk of product recalls, delays, or market withdrawals due to impurity issues.
Explore : https://aquigenbio.com/products/impurity-standards/nitroso/
Related Product - Riociguat Nitroso USP Related Compound C
Alongside Riociguat N-Nitroso Des Formyl Impurity, we also offer Riociguat Nitroso USP Related Compound C - another essential reference standard in Riociguat impurity profiling. Using multiple related standards ensures a comprehensive understanding of the nitroso impurity landscape, enabling better control strategies in manufacturing.
By incorporating both impurities into analytical workflows, laboratories can:
Compare retention times for better chromatographic resolution
Perform cross-validation between methods
Ensure that both major and trace-level impurities are identified and quantified
Explore : https://aquigenbio.com/product/riociguat-nitroso-usp-related-compound-c/
Why Choose Aquigen Bio for Nitroso Impurity Standards?
At Aquigen Bio, we understand that in pharmaceutical research and production, accuracy is non-negotiable. Our nitroso impurity standards are trusted by analytical labs, pharmaceutical companies, and CROs worldwide for their:
High purity and traceability - Produced under strict quality control to ensure reliability
Comprehensive documentation - Detailed CoAs, safety data, and spectral analysis provided
Custom synthesis capability - Ability to develop unique impurity standards not available in the market
Global supply network - Reliable, timely delivery across multiple geographies
Regulatory expertise - Products aligned with USFDA, EMA, and ICH guidelines
Storage, Handling & Safety
Riociguat N-Nitroso Des Formyl Impurity should be stored under recommended conditions as mentioned in the CoA, typically in a cool, dry, and light-protected environment. Handling must follow standard laboratory safety protocols, including the use of PPE, fume hoods, and proper waste disposal methods in compliance with local regulations.
Supporting Analytical Excellence
With regulatory agencies increasingly scrutinizing nitroso impurities, having the right analytical tools is vital. The Riociguat N-Nitroso Des Formyl Impurity from Aquigen Bio empowers laboratories to meet these demands with precision and confidence.
Our commitment extends beyond just supplying high-quality standards - we aim to be your partner in analytical success. Whether you are developing stability-indicating methods, performing release testing, or investigating root causes of contamination, our products help streamline your workflow and improve data reliability.
Contact:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen Bio Sciences
Aquigen Bio Sciences is at the forefront of pharmaceutical excellence, serving as the leading resource for Riociguat impurity standards in India. With a strong commitment to safety, innovation, and regulatory compliance, the organization specializes in impurity profiling, synthesis, and analysis, empowering pharmaceutical manufacturers globally with premier standards. Aquigen Bio Sciences' dedication to cutting-edge research ensures it remains a trusted partner in refining quality benchmarks across the pharmaceutical landscape.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Riociguat N-Nitroso Des Formyl Impurity - Reliable Reference Standard for Advanced Pharmaceutical Analysis here
News-ID: 4141956 • Views: …
More Releases from Aquigen Bio Sciences

Elevate Pharmaceutical R&D with Aquigen BioSciences' Precision‐Grade Flibanser …
Flibanserin Impurity B is a reference standard used in pharmaceutical research and development. It is primarily applied during the analysis and validation of drug substances to identify, quantify, and control impurities that may be present in the final product. This impurity is associated with the parent compound, Flibanserin, a medication approved for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women.
Aquigen BioSciences offers Flibanserin Impurity B as a…

Estradiol Valerate EP Impurity A - Premium Reference Standard for Analytical Dev …
Estradiol Valerate EP Impurity A is a high-quality reference standard designed to meet the stringent requirements of pharmaceutical research, method validation, and quality control processes.
Explore Estradiol Valerate EP Impurity A :
https://aquigenbio.com/product/estradiol-valerate-ep-impurity-a/
Manufactured and characterized with precision, this impurity standard supports laboratories and manufacturers in achieving consistent, reliable, and reproducible results in critical analytical workflows.
With its exceptional purity and accurate characterization, Estradiol Valerate EP Impurity A plays a vital role…

High-Purity N-Nitroso Betahistine D3 for Precise Pharmaceutical Analysis | Deute …
Product Overview
N-Nitroso Betahistine D3 is a premium deuterated nitrosamine impurity standard, specifically developed for precise analytical testing in pharmaceutical laboratories. This reference standard is widely used for analytical method development, validation, and quality control processes to meet stringent regulatory guidelines. With exceptional purity, complete documentation, and reliable traceability, it is ideal for research, development, and compliance applications.
https://aquigenbio.com/product/n-nitroso-betahistine-d3/
Key Features and Benefits
Deuterated Design for Precision: The incorporation of deuterium improves mass spectrometric…

Aquigen Bio Strengthens Pharmaceutical Research with High-Purity Icatibant Impur …
Aquigen Bio, a trusted supplier of pharmaceutical reference standards, today announced the expansion of its Icatibant Impurity Standards portfolio, designed to support drug developers, analytical laboratories, and research organizations with reliable materials for impurity profiling and quality control.
Icatibant, a selective bradykinin B2 receptor antagonist, is widely used in the treatment of hereditary angioedema (HAE). Given its peptide-based structure, Icatibant is prone to the formation of impurities during synthesis and storage.…
More Releases for Riociguat
Chronic Thromboembolic Pulmonary Hypertension Therapeutics Market 2034: Clinical …
(Albany, USA) DelveInsight's "Chronic Thromboembolic Pulmonary Hypertension Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Chronic Thromboembolic Pulmonary Hypertension, historical and forecasted epidemiology as well as the Chronic Thromboembolic Pulmonary Hypertension market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
The Chronic Thromboembolic Pulmonary Hypertension market report provides current treatment practices, emerging drugs, the market share of the individual…
Chronic Thromboembolic Pulmonary Hypertension Drugs Market 2034: Clinical Trials …
(Albany, USA) DelveInsight's "Chronic Thromboembolic Pulmonary Hypertension Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Chronic Thromboembolic Pulmonary Hypertension, historical and forecasted epidemiology as well as the Chronic Thromboembolic Pulmonary Hypertension market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
The Chronic Thromboembolic Pulmonary Hypertension market report provides current treatment practices, emerging drugs, the market share of the individual…
Chronic Thromboembolic Pulmonary Hypertension Treatment Market 2034: EMA, PDMA, …
(Albany, USA) DelveInsight's "Chronic Thromboembolic Pulmonary Hypertension Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Chronic Thromboembolic Pulmonary Hypertension, historical and forecasted epidemiology as well as the Chronic Thromboembolic Pulmonary Hypertension market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
The Chronic Thromboembolic Pulmonary Hypertension market report provides current treatment practices, emerging drugs, the market share of the individual…
Chronic Pulmonary Hypertension Market Size in the 7MM was ~USD 28,290 million in …
DelveInsight's "Chronic Pulmonary Hypertension Market Insights, Epidemiology, and Market Forecast - 2034" report delivers an in-depth understanding, historical and forecasted epidemiology, as well as the Chronic Pulmonary Hypertension market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Chronic Pulmonary Hypertension Market is an evolving segment of the global healthcare landscape, driven by the increasing Chronic Pulmonary Hypertension prevalence of the…
Chronic Pulmonary Hypertension Market Size in the 7MM was ~USD 28,290 million in …
DelveInsight's "Chronic Pulmonary Hypertension Market Insights, Epidemiology, and Market Forecast - 2034" report delivers an in-depth understanding, historical and forecasted epidemiology, as well as the Chronic Pulmonary Hypertension market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Chronic Pulmonary Hypertension Market is an evolving segment of the global healthcare landscape, driven by the increasing Chronic Pulmonary Hypertension prevalence of the disorder…
Hypertension Pipeline Outlook Report 2024 (Updated)
DelveInsight's, "Hypertension Pipeline Insight 2024" report provides comprehensive insights about 100+ companies and 100+ pipeline drugs in the Hypertension pipeline landscape. It covers the Hypertension pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Hypertension pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key Takeaways from the Hypertension Pipeline Report
• DelveInsight's Hypertension…