Press release
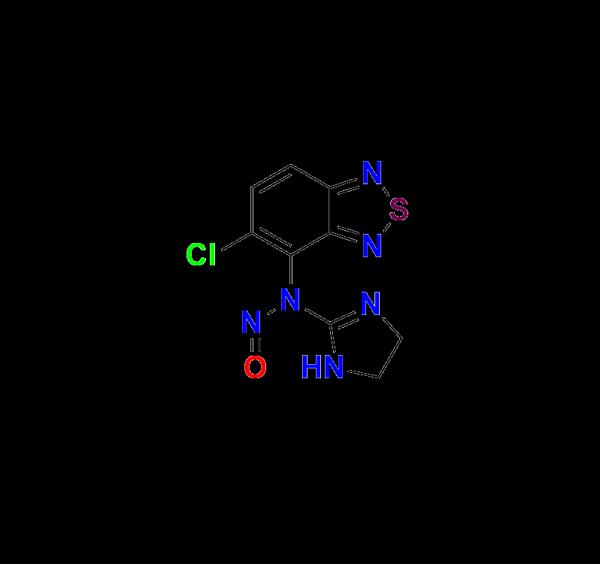
Tizanidine Nitroso Impurity 1 - High-Purity Reference Standard for Reliable Impurity Profiling, Regulatory Compliance, and Safer Tizanidine Drug Development | Aquigen Bio Nitroso Impurity Standards
In the highly regulated world of pharmaceutical manufacturing, impurity profiling plays a critical role in ensuring drug safety, efficacy, and regulatory compliance. Among the wide range of impurity reference standards required during API development and validation, Tizanidine Nitroso Impurity 1 stands out as a vital compound for researchers and manufacturers working on Tizanidine-based formulations.At Aquigen Bio, we are proud to offer Tizanidine Nitroso Impurity 1, a high-purity reference standard meticulously synthesized and validated to meet the rigorous demands of pharmaceutical quality control, analytical method development, and regulatory filing processes.
What is Tizanidine Nitroso Impurity 1?
https://aquigenbio.com/product/tizanidine-nitroso-impurity-1/
Tizanidine is a centrally acting α2-adrenergic agonist commonly prescribed as a muscle relaxant to treat conditions like multiple sclerosis and spinal cord injuries. During the synthesis or degradation of the Tizanidine active pharmaceutical ingredient (API), nitroso impurities may be formed, either through unintended reactions or environmental exposure.
Tizanidine Nitroso Impurity 1 is one such potential impurity that must be identified, monitored, and controlled as per international guidelines (ICH M7, Q3A, Q3B). Nitroso compounds are under heightened scrutiny due to their potential genotoxicity and carcinogenic risk. As a result, the ability to detect even trace amounts of Nitroso Impurity 1 is essential during pharmaceutical development and batch release.
Why Tizanidine Nitroso Impurity 1 Matters in Drug Safety
The importance of Tizanidine Nitroso Impurity 1 is rooted in its impact on patient safety and drug approval:
Regulatory Compliance: Regulatory bodies like the USFDA, EMA, and CDSCO now mandate stringent control of nitrosamine-related impurities. Tizanidine Nitroso Impurity 1 falls within this category.
Risk Assessment & Mitigation: Its inclusion in risk assessments allows pharmaceutical manufacturers to confidently demonstrate product safety.
Explore Nitroso Impurities
https://aquigenbio.com/products/impurity-standards/nitroso/
Analytical Method Development: With the availability of certified reference standards, QC teams can develop and validate accurate testing methods for routine batch analysis.
The availability of high-purity, well-characterized impurity standards from reliable sources such as Aquigen Bio enables seamless regulatory submissions and improves the overall safety profile of the pharmaceutical product.
Aquigen Bio: Trusted Partner for Nitroso Impurity Standards
At Aquigen Bio, we understand that access to certified impurity standards is critical to the drug development lifecycle. Our extensive range of nitroso impurities includes:
Tizanidine Nitroso Impurity 1
Tizanidine Nitroso Impurity 2
Tizanidine Nitroso Impurity 3
https://aquigenbio.com/product/tizanidine-nitroso-impurity-3/
These are part of our broader Nitroso Impurity Standards category, specially curated to meet industry needs for genotoxic impurity profiling.
Each of our impurity products undergoes:
Analytical characterization via NMR, IR, Mass, and HPLC.
Certificate of Analysis (CoA) with traceable documentation.
Batch-to-batch consistency to support long-term research needs.
Custom pack sizes and prompt global shipping to support urgent R&D timelines.
Applications of Tizanidine Nitroso Impurity 1 in Pharma R&D
Analytical Method Development & Validation
Impurity 1 serves as a reference for developing stability-indicating methods, validating HPLC/LC-MS techniques, and preparing calibration curves for quantification.
Stability Studies
During forced degradation or accelerated stability studies, the detection of Tizanidine Nitroso Impurity 1 helps determine degradation pathways and product shelf-life.
Regulatory Submissions
ICH guidelines require full disclosure and documentation of all potential nitroso impurities in APIs. Having a validated standard like Tizanidine Nitroso Impurity 1 ensures data accuracy and regulatory readiness.
Toxicological Evaluation
In silico and in vitro studies require well-characterized impurities to assess their genotoxicity potential - vital for justifying permitted daily exposure (PDE) limits.
Why Choose Aquigen Bio?
Aquigen Bio is a pioneer in supplying high-purity pharmaceutical impurity reference standards to leading manufacturers and CROs worldwide. Our R&D scientists and analytical chemists follow strict quality protocols to deliver reliable standards that support:
Faster drug development timelines
Regulatory risk mitigation
Cost-effective and reproducible impurity profiling
Our customers appreciate the transparency of our analytical reports, responsiveness to technical queries, and a robust logistics framework that ensures timely delivery in compliance with global transport regulations.
Additional Tizanidine Nitroso Impurities Available
Along with Impurity 1, Aquigen Bio also offers:
Tizanidine Nitroso Impurity 2:
https://aquigenbio.com/product/tizanidine-nitroso-impurity-2/
Ideal for orthogonal method development and related substance analysis.
Tizanidine Nitroso Impurity 3: Useful for confirming degradation patterns under various stress conditions.
Together, these standards offer a comprehensive impurity profiling toolkit for your Tizanidine projects.
Global Delivery & Custom Orders
Whether you are conducting early-stage R&D or preparing for regulatory submission, Aquigen Bio can support your needs with:
Custom synthesis of rare impurities
Flexible pack sizes
Secure packaging and fast global delivery
Dedicated customer support for documentation and technical questions
Our team ensures that your lab gets the exact material you need - with full traceability and compliance assurance.
Contact:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen Bio Sciences
Aquigen Bio Sciences is at the forefront of pharmaceutical excellence, serving as the leading resource for Gefitinib impurity standards in India. With a strong commitment to safety, innovation, and regulatory compliance, the organization specializes in impurity profiling, synthesis, and analysis, empowering pharmaceutical manufacturers globally with premier standards. Aquigen Bio Sciences' dedication to cutting-edge research ensures it remains a trusted partner in refining quality benchmarks across the pharmaceutical landscape.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Tizanidine Nitroso Impurity 1 - High-Purity Reference Standard for Reliable Impurity Profiling, Regulatory Compliance, and Safer Tizanidine Drug Development | Aquigen Bio Nitroso Impurity Standards here
News-ID: 4133311 • Views: …
More Releases from Aquigen Bio Sciences

Elevate Pharmaceutical R&D with Aquigen BioSciences' Precision‐Grade Flibanser …
Flibanserin Impurity B is a reference standard used in pharmaceutical research and development. It is primarily applied during the analysis and validation of drug substances to identify, quantify, and control impurities that may be present in the final product. This impurity is associated with the parent compound, Flibanserin, a medication approved for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women.
Aquigen BioSciences offers Flibanserin Impurity B as a…

Estradiol Valerate EP Impurity A - Premium Reference Standard for Analytical Dev …
Estradiol Valerate EP Impurity A is a high-quality reference standard designed to meet the stringent requirements of pharmaceutical research, method validation, and quality control processes.
Explore Estradiol Valerate EP Impurity A :
https://aquigenbio.com/product/estradiol-valerate-ep-impurity-a/
Manufactured and characterized with precision, this impurity standard supports laboratories and manufacturers in achieving consistent, reliable, and reproducible results in critical analytical workflows.
With its exceptional purity and accurate characterization, Estradiol Valerate EP Impurity A plays a vital role…

High-Purity N-Nitroso Betahistine D3 for Precise Pharmaceutical Analysis | Deute …
Product Overview
N-Nitroso Betahistine D3 is a premium deuterated nitrosamine impurity standard, specifically developed for precise analytical testing in pharmaceutical laboratories. This reference standard is widely used for analytical method development, validation, and quality control processes to meet stringent regulatory guidelines. With exceptional purity, complete documentation, and reliable traceability, it is ideal for research, development, and compliance applications.
https://aquigenbio.com/product/n-nitroso-betahistine-d3/
Key Features and Benefits
Deuterated Design for Precision: The incorporation of deuterium improves mass spectrometric…

Aquigen Bio Strengthens Pharmaceutical Research with High-Purity Icatibant Impur …
Aquigen Bio, a trusted supplier of pharmaceutical reference standards, today announced the expansion of its Icatibant Impurity Standards portfolio, designed to support drug developers, analytical laboratories, and research organizations with reliable materials for impurity profiling and quality control.
Icatibant, a selective bradykinin B2 receptor antagonist, is widely used in the treatment of hereditary angioedema (HAE). Given its peptide-based structure, Icatibant is prone to the formation of impurities during synthesis and storage.…
More Releases for Nitroso
Riociguat N-Nitroso Des Formyl Impurity - Reliable Reference Standard for Advanc …
Riociguat N-Nitroso Des Formyl Impurity is a premium-quality analytical reference material developed to support research, testing, and quality control in the pharmaceutical industry.
As regulatory guidelines tighten worldwide, accurate detection, quantification, and control of nitroso impurities have become an essential part of drug development and manufacturing.
This compound plays a vital role in ensuring that formulations containing Riociguat meet the highest safety and compliance standards.
The Growing Importance of Nitroso Impurity…
Aquigen Bio Unveils N-Nitroso Felodipine: A New Benchmark in Pharmaceutical Refe …
In an era where drug safety and efficacy are paramount, the pharmaceutical industry faces increasing pressure to develop robust analytical methods that ensure the purity and quality of medicinal products. N-Nitroso Felodipine is specifically engineered to address these challenges. It serves as an indispensable tool for Analytical Method Development (AMD) and Analytical Method Validation (AMV), providing a reliable benchmark against which new and existing analytical procedures can be assessed for…
N-Nitroso Impurities: Carcinogenic Risks, Analytical Challenges, and Compliance …
N-Nitroso impurities are emerging as a significant concern in the pharmaceutical industry due to their potential carcinogenicity. These compounds, formed during the manufacturing or storage of drug products, have raised alarms among regulatory agencies across the globe. Pharmaceutical companies face mounting pressure to identify, analyze, and mitigate these impurities through stringent guidelines to ensure patient safety.
The serious risks posed by N-Nitroso compounds stem from their ability to induce genetic mutations…
Bumetanide Nitroso Impurity: Mitigating Compliance Risks and Integrating Innovat …
Bumetanide Nitroso Impurity has emerged as a significant concern in the pharmaceutical industry, particularly for companies involved in the development and manufacturing of medications used to manage edema. As awareness grows about the potential risks associated with nitroso impurities, regulatory agencies worldwide are increasing scrutiny over pharmaceutical products. This press release aims to address the challenges posed by Bumetanide Nitroso Impurity, outline the regulatory landscape, and highlight innovative practices that…
Sumatriptan Nitroso Impurity: Navigating Compliance Challenges and Implementing …
Sumatriptan Nitroso Impurity has become a pressing concern in the pharmaceutical industry, as companies grapple with significant compliance challenges related to these potentially harmful contaminants. As Sumatriptan, a widely prescribed medication for migraine relief, comes under increasing scrutiny from regulatory bodies, pharmaceutical companies must navigate a complex landscape to ensure product safety and adhere to evolving regulatory standards.
What is the issue?
Nitroso impurities have emerged as a pressing concern within the…
N-Nitroso-N-methylurea Market Expected to Develop by 2028 with COVID-19 Analysis
This N-Nitroso-N-methylurea market research report depicts evaluation methods, promotion methods, major updates, innovations and inventions. It enables business participants to track business profits, trends and latest market trends. It becomes easy for industry owners to set company goal through this market analysis report. The market statistics are obtained from secondary resources. Moreover, analysts perform detailed market study making use of a regression technique for attaining particular data about segment as…