Press release
Ceritinib Nitroso Impurity 1: Enhancing Cancer Therapy Safety with Aquigen Bio Sciences' High-Quality Standards and Solutions for Regulatory Compliance, Ensuring Patient Safety and Efficacy in ALK-Positive Non-Small Cell Lung Cancer Treatments
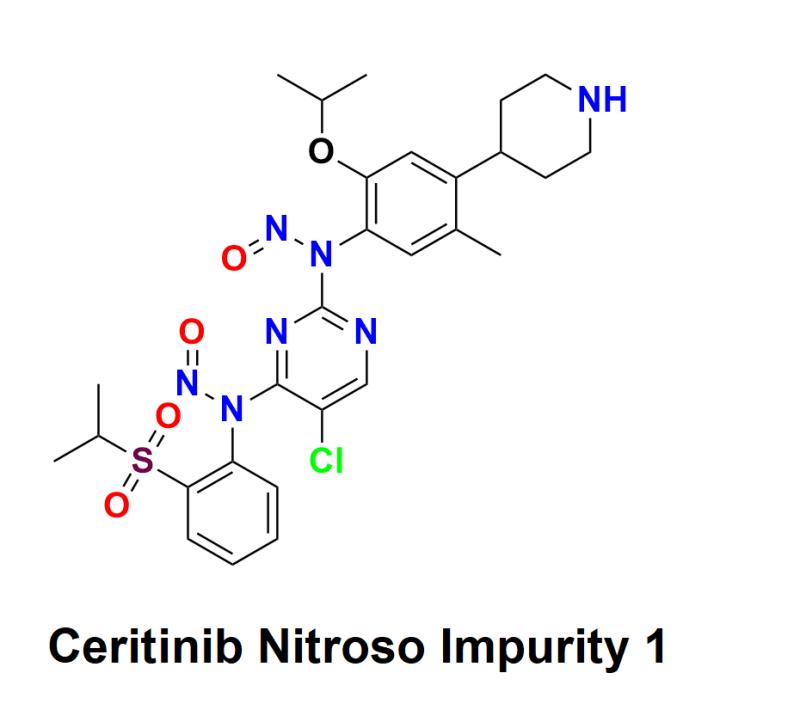
Ceritinib Nitroso Impurity 1 is an impurity associated with ceritinib, a targeted therapy medication primarily used in the treatment of non-small cell lung cancer (NSCLC). Ceritinib is an oral medication that inhibits anaplastic lymphoma kinase (ALK) and is prescribed for patients who are ALK-positive. The identification and quantification of ceritinib nitroso impurity 1 are crucial for maintaining the safety, efficacy, and regulatory compliance of pharmaceutical products containing ceritinib.Learn more about Ceritinib Nitroso Impurity 1: https://aquigenbio.com/product/ceritinib-nitroso-impurity-1-2/
Understanding Ceritinib Nitroso Impurity 1:
Ceritinib Nitroso Impurity 1 is classified as a nitroso impurity, which refers to a group of compounds that contain the nitroso functional group (-N=O). Nitroso impurities can form during the synthesis and manufacturing processes of pharmaceutical compounds and can have varying degrees of toxicity. Specifically, ceritinib nitroso impurity 1 arises from the chemical reactions involved in the production of ceritinib, particularly under conditions that can promote nitrosation-a reaction in which nitrosamines are formed.
These nitroso impurities have garnered significant attention due to their potential carcinogenic properties, necessitating rigorous testing and monitoring to ensure the safety of patients receiving ceritinib therapy. Regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established guidelines to limit the acceptable levels of nitroso impurities in pharmaceutical products, ensuring that patients are protected from potential health risks.
The Need for Ceritinib Nitroso Impurity 1:
The necessity of addressing ceritinib nitroso impurity 1 lies in the imperative to safeguard patient health. As more patients turn to targeted therapies like ceritinib for cancer treatment, understanding and controlling the presence of impurities is essential.
Get in Touch with Us - https://aquigenbio.com/contact-us/
1. Patient Safety:
The primary concern in pharmaceuticals is patient safety. Ceritinib is a critical treatment for patients with ALK-positive NSCLC, and the presence of nitroso impurities poses potential risks. Studies have suggested that exposure to nitroso compounds can lead to the formation of harmful byproducts in the body, potentially increasing the risk of cancer. This risk underscores the importance of detecting and quantifying ceritinib nitroso impurity 1 in any formulation, as it helps manufacturers ensure that their products remain within the acceptable limits set by health authorities.
2. Regulatory Compliance:
Pharmaceutical companies are mandated to comply with stringent regulations governing the manufacturing and quality control of their products. The detection and analysis of ceritinib nitroso impurity 1 are vital for meeting the quality assurance requirements outlined by regulatory agencies. Companies that fail to adequately monitor nitroso impurities may face penalties, product recalls, and damage to their reputations. Thus, implementing robust testing protocols for ceritinib nitroso impurity 1 not only helps ensure patient safety but also facilitates compliance with regulatory standards.
3. Efficacy of Treatment:
Impurities can impact the overall effectiveness of the medication. Ceritinib's therapeutic benefits rely on its ability to inhibit ALK effectively; however, the presence of impurities like ceritinib nitroso impurity 1 can interfere with this action. High levels of impurities may affect drug stability and bioavailability, potentially leading to reduced efficacy and poor patient outcomes. Therefore, thorough testing and quality assurance practices are essential to maintain the therapeutic profile of ceritinib.
4. Public Trust:
The pharmaceutical industry operates on trust. Patients need to have confidence in the medications they receive. Transparency regarding the presence of impurities, including ceritinib nitroso impurity 1, plays a significant role in building and maintaining that trust. By conducting thorough assessments and openly communicating the safety and quality of their products, pharmaceutical companies can foster a positive relationship with healthcare professionals and patients alike.
Conclusion:
Ceritinib Nitroso Impurity 1 represents a critical aspect of ensuring the safety and efficacy of ceritinib in the treatment of non-small cell lung cancer. The need to identify, quantify, and control this impurity is paramount to protect patient health, maintain regulatory compliance, and preserve the efficacy of this vital cancer therapy.
As the healthcare landscape continues to evolve, ongoing research and advancements in analytical techniques will play a significant role in monitoring and managing nitroso impurities, including ceritinib nitroso impurity 1. Pharmaceutical companies must remain vigilant in their quality control measures and commit to transparency in their operations, ensuring that they prioritize patient safety above all else.
For more information about ceritinib and its impurities, please contact Aquigen Bio Sciences, a trusted resource for ceritinib nitroso impurity 1. Aquigen Bio Sciences is a leader in impurity standards and offers comprehensive solutions for manufacturers looking to navigate the complexities of pharmaceutical safety.
Similar Trending Products:
1) N-Nitroso Ropinirole: https://aquigenbio.com/product/n-nitroso-ropinirole/
2) N-Nitroso Bromocriptine: https://aquigenbio.com/product/n-nitroso-bromocriptine/
3) N-Nitroso -N-Demethyl Roxithromycin: https://aquigenbio.com/product/n-nitroso-n-demethyl-roxithromycin/
Contact Us:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen Bio Sciences:
Aquigen Bio Sciences is a renowned contract research organization based in Pune, India, specializing in impurity standards and comprehensive solutions for the pharmaceutical industry. With a focus on advancing pharmaceutical safety and compliance, Aquigen provides expert guidance on identifying, quantifying, and managing impurities in various drug formulations. Their commitment to quality and regulatory excellence positions them as a trusted partner for manufacturers striving to meet stringent safety standards and improve patient outcomes.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Ceritinib Nitroso Impurity 1: Enhancing Cancer Therapy Safety with Aquigen Bio Sciences' High-Quality Standards and Solutions for Regulatory Compliance, Ensuring Patient Safety and Efficacy in ALK-Positive Non-Small Cell Lung Cancer Treatments here
News-ID: 3719592 • Views: …
More Releases from Aquigen Biosciences
Precision Standards for Oncology Research: Exploring Abemaciclib Impurity 1 and …
In the ever-evolving field of targeted cancer therapy, Abemaciclib has emerged as a pivotal agent in the treatment of hormone receptor-positive (HR+), HER2-negative advanced or metastatic breast cancer. As researchers and pharmaceutical developers continue to innovate in oncology, the importance of impurity profiling and the availability of reliable Abemaciclib impurity standards has never been greater.
At the forefront of pharmaceutical impurity standards, Aquigen Bio is proud to support global manufacturers, CROs,…
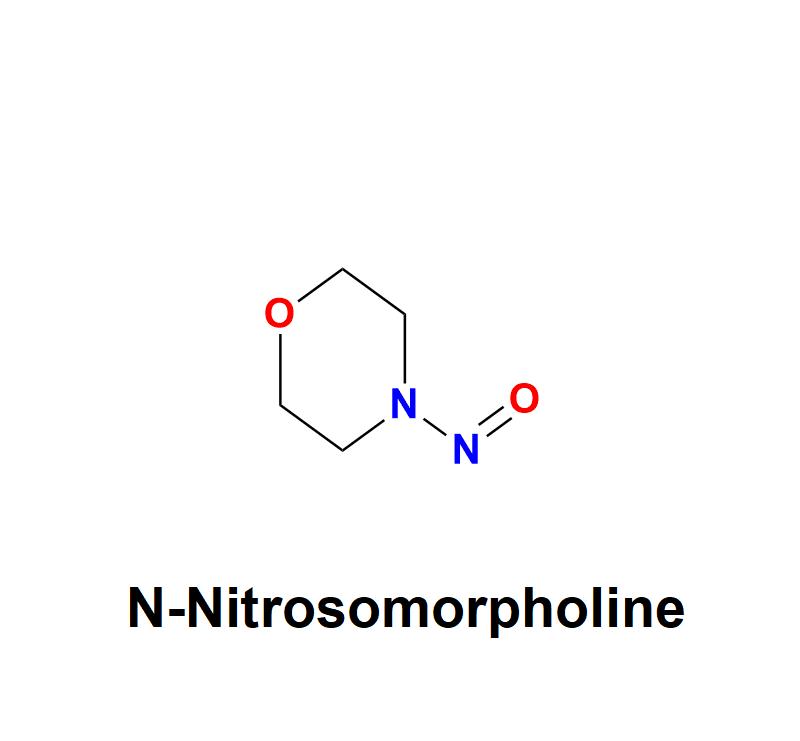
N-Nitrosomorpholine: Addressing Pharmaceutical Safety Challenges with Aquigen Bi …
N-Nitrosomorpholine, a compound belonging to the nitrosamine family, has garnered significant attention in the pharmaceutical and healthcare industries due to its potential carcinogenic risks. This chemical impurity, often found as a byproduct in manufacturing processes, poses serious challenges to drug safety and human health, necessitating stringent monitoring and control measures from pharmaceutical companies.
Learn more about N-Nitrosomorpholine: https://aquigenbio.com/product/n-nitrosomorpholine/
Understanding N-Nitrosomorpholine:
N-Nitrosomorpholine is a nitrosamine impurity characterized by its chemical structure, which includes…
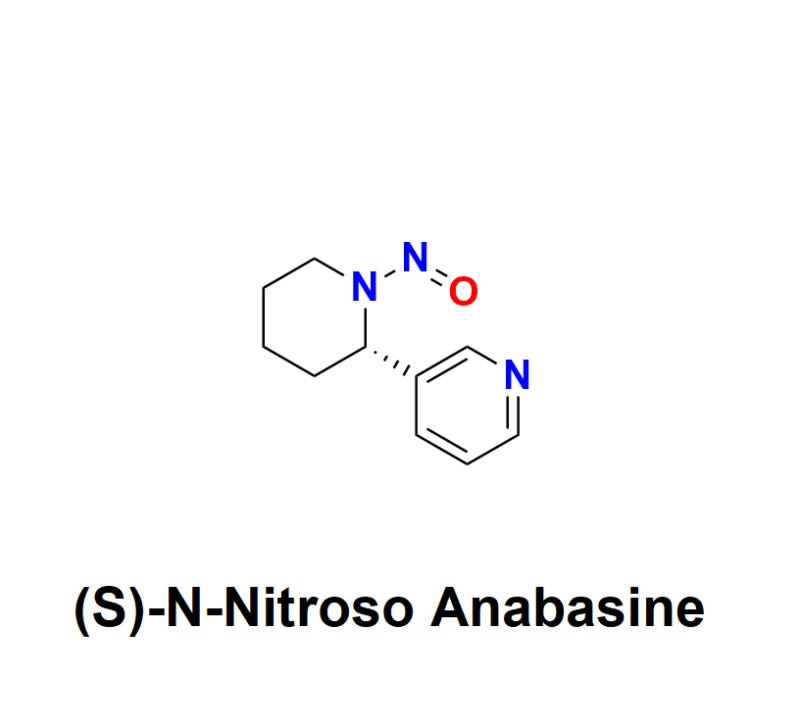
(S)-N-Nitroso Anabasine: Understanding the Risks, Regulatory Challenges, and How …
(S)-N-Nitroso Anabasine, a nitrosamine impurity, has raised significant safety concerns within the pharmaceutical industry. Recognized as a probable human carcinogen, this impurity has become a focal point for global regulatory agencies and manufacturers alike, urging a renewed emphasis on detection, prevention, and management.
Learn more about (S)-N-Nitroso Anabasine: https://aquigenbio.com/product/s-n-nitroso-anabasine/
What Is (S)-N-Nitroso Anabasine?
(S)-N-Nitroso Anabasine belongs to the family of nitrosamines, compounds formed through a chemical reaction known as nitrosation. This…
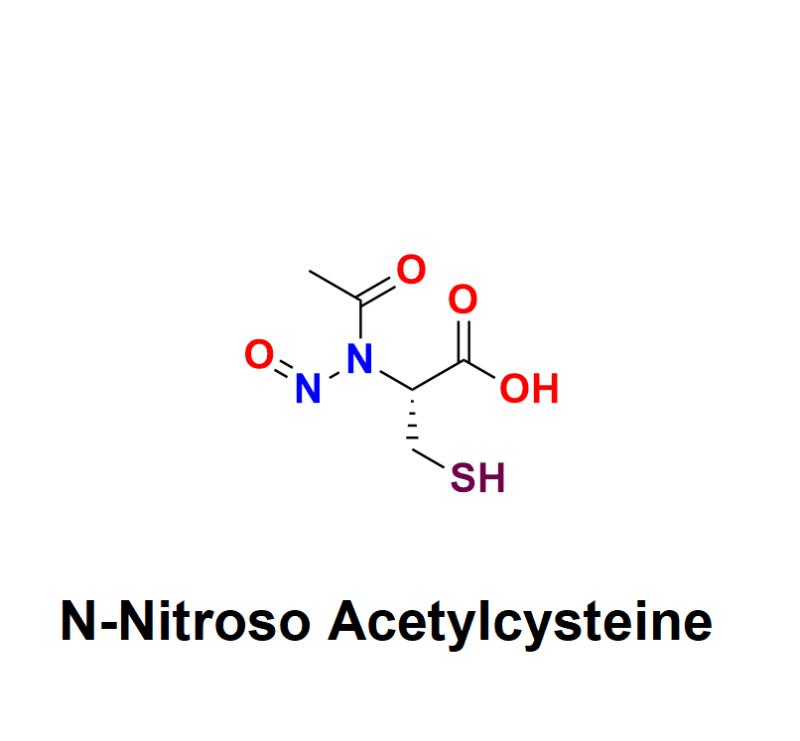
N-Nitroso Acetylcysteine: A Critical Concern in Pharmaceuticals - Exploring Haza …
N-Nitroso Acetylcysteine has emerged as a critical topic of concern in the pharmaceutical industry. As a member of the nitrosamine family, it is a potential impurity that poses significant health risks, including carcinogenicity, even in trace amounts. With increasing regulatory scrutiny on nitrosamine impurities, pharmaceutical manufacturers must address the presence of compounds like N-Nitroso Acetylcysteine to protect public health and ensure compliance with global standards.
Learn more about N-Nitroso Acetylcysteine: https://aquigenbio.com/product/n-nitroso-acetylcysteine/…
More Releases for Nitroso
Riociguat N-Nitroso Des Formyl Impurity - Reliable Reference Standard for Advanc …
Riociguat N-Nitroso Des Formyl Impurity is a premium-quality analytical reference material developed to support research, testing, and quality control in the pharmaceutical industry.
As regulatory guidelines tighten worldwide, accurate detection, quantification, and control of nitroso impurities have become an essential part of drug development and manufacturing.
This compound plays a vital role in ensuring that formulations containing Riociguat meet the highest safety and compliance standards.
The Growing Importance of Nitroso Impurity…
Tizanidine Nitroso Impurity 1 - High-Purity Reference Standard for Reliable Impu …
In the highly regulated world of pharmaceutical manufacturing, impurity profiling plays a critical role in ensuring drug safety, efficacy, and regulatory compliance. Among the wide range of impurity reference standards required during API development and validation, Tizanidine Nitroso Impurity 1 stands out as a vital compound for researchers and manufacturers working on Tizanidine-based formulations.
At Aquigen Bio, we are proud to offer Tizanidine Nitroso Impurity 1, a high-purity reference standard meticulously…
Aquigen Bio Unveils N-Nitroso Felodipine: A New Benchmark in Pharmaceutical Refe …
In an era where drug safety and efficacy are paramount, the pharmaceutical industry faces increasing pressure to develop robust analytical methods that ensure the purity and quality of medicinal products. N-Nitroso Felodipine is specifically engineered to address these challenges. It serves as an indispensable tool for Analytical Method Development (AMD) and Analytical Method Validation (AMV), providing a reliable benchmark against which new and existing analytical procedures can be assessed for…
N-Nitroso Impurities: Carcinogenic Risks, Analytical Challenges, and Compliance …
N-Nitroso impurities are emerging as a significant concern in the pharmaceutical industry due to their potential carcinogenicity. These compounds, formed during the manufacturing or storage of drug products, have raised alarms among regulatory agencies across the globe. Pharmaceutical companies face mounting pressure to identify, analyze, and mitigate these impurities through stringent guidelines to ensure patient safety.
The serious risks posed by N-Nitroso compounds stem from their ability to induce genetic mutations…
Bumetanide Nitroso Impurity: Mitigating Compliance Risks and Integrating Innovat …
Bumetanide Nitroso Impurity has emerged as a significant concern in the pharmaceutical industry, particularly for companies involved in the development and manufacturing of medications used to manage edema. As awareness grows about the potential risks associated with nitroso impurities, regulatory agencies worldwide are increasing scrutiny over pharmaceutical products. This press release aims to address the challenges posed by Bumetanide Nitroso Impurity, outline the regulatory landscape, and highlight innovative practices that…
Sumatriptan Nitroso Impurity: Navigating Compliance Challenges and Implementing …
Sumatriptan Nitroso Impurity has become a pressing concern in the pharmaceutical industry, as companies grapple with significant compliance challenges related to these potentially harmful contaminants. As Sumatriptan, a widely prescribed medication for migraine relief, comes under increasing scrutiny from regulatory bodies, pharmaceutical companies must navigate a complex landscape to ensure product safety and adhere to evolving regulatory standards.
What is the issue?
Nitroso impurities have emerged as a pressing concern within the…