Press release
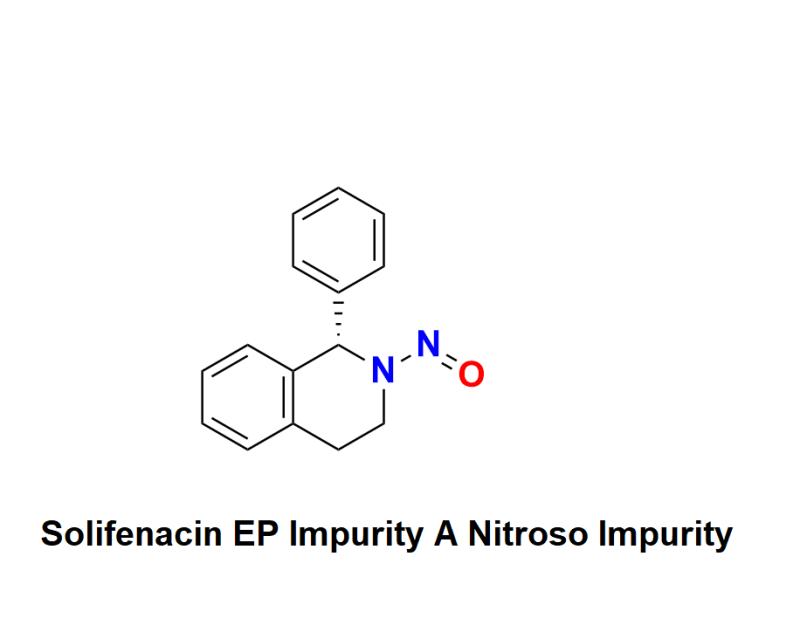
Solifenacin EP Impurity A Nitroso Impurity: Navigating Compliance Risks, Regulatory Challenges, and Innovative Testing Solutions with Aquigen Bio Sciences to Safeguard Patient Safety and Uphold Pharmaceutical Standards
Solifenacin EP Impurity A Nitroso Impurity has surfaced as a pressing concern in the pharmaceutical industry, raising significant alarms among manufacturers and regulatory bodies. This press release aims to examine the challenges presented by Solifenacin EP Impurity A Nitroso Impurity, outline the shifting regulatory landscape, and highlight the innovative testing solutions available to help pharmaceutical companies reduce compliance risks while safeguarding patient safety.The Importance of Solifenacin in Treating Overactive Bladder:
Solifenacin is a well-established anticholinergic medication prescribed for managing symptoms of overactive bladder, such as urgency and increased frequency. While it plays a crucial role in improving patients' quality of life, the identification of nitroso impurities, particularly Solifenacin EP Impurity A, raises serious concerns about patient safety and complicates compliance efforts for manufacturers.
Learn more about Solifenacin EP Impurity A Nitroso Impurity: https://aquigenbio.com/product/solifenacin-ep-impurity-a-nitroso-impurity/
Challenges Posed By Solifenacin EP Impurity A Nitroso Impurity:
Nitroso impurities are recognized as potential carcinogens and can arise during the synthesis or storage of pharmaceutical products. These impurities can be challenging to detect and quantify, posing unique challenges for manufacturers. The emergence of Solifenacin EP Impurity A Nitroso Impurity underscores the urgent need for comprehensive risk management strategies in the pharmaceutical sector.
The presence of nitroso impurities in Solifenacin raises substantial safety concerns. As regulatory agencies increase their oversight, pharmaceutical companies face enhanced scrutiny, which can result in product recalls, regulatory penalties, and reputational damage.
Navigating the Regulatory Landscape:
The regulatory framework governing Solifenacin EP Impurity A Nitroso Impurity has evolved significantly, reflecting a growing awareness of the dangers posed by nitroso contaminants. Regulatory bodies, such as the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency), have issued extensive guidelines to assist pharmaceutical manufacturers in identifying, assessing, and managing these impurities. These guidelines emphasize the need for rigorous testing protocols tailored for Solifenacin, detailed risk assessments, and thorough documentation to ensure compliance with stringent safety standards.
Pharmaceutical companies must take proactive measures in their compliance strategies to mitigate risks related to nitroso impurities. Failure to adequately address these issues can lead to severe consequences, including regulatory penalties and loss of public trust in product safety.
Get in Touch with Us - https://aquigenbio.com/contact-us/
Tackling Challenges and Enhancing Compliance:
To effectively address the complexities associated with Solifenacin EP Impurity A Nitroso Impurity and other nitroso contaminants, pharmaceutical companies can adopt various strategies designed to bolster compliance and maintain safety standards:
1. State-of-the-Art Analytical Techniques: Utilizing advanced methodologies, such as liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry (GC-MS), allows manufacturers to detect and quantify nitroso impurities even at trace levels.
2. Thorough Risk Assessments: Conducting comprehensive evaluations of raw materials, manufacturing processes, and storage conditions is essential for identifying potential sources of nitroso impurity formation and developing effective control measures.
3. Interdepartmental Collaboration: Promoting collaboration across departments-such as quality assurance, regulatory affairs, and research and development-can strengthen compliance frameworks and ensure alignment with regulatory expectations.
4. Continuous Monitoring Practices: Implementing ongoing monitoring and testing protocols throughout the manufacturing process enables early identification of nitroso impurities, ensuring adherence to established safety standards.
5. Robust Supplier Quality Management: Establishing stringent quality management systems for suppliers and conducting regular audits helps maintain the quality of raw materials and active pharmaceutical ingredients (APIs), reducing the risk of contamination.
Commitment to Patient Safety and Trust:
Patient safety is paramount in the pharmaceutical sector, and the presence of nitroso impurities necessitates immediate action to mitigate risks. By implementing effective strategies and fostering a culture of compliance, manufacturers can demonstrate their dedication to patient safety while enhancing the overall quality of their products.
Conclusion:
As the pharmaceutical industry confronts the complexities of nitroso contaminants, addressing compliance risks associated with Solifenacin EP Impurity A Nitroso Impurity is crucial. By integrating innovative testing methodologies, conducting thorough risk assessments, and encouraging collaboration, companies can uphold safety and regulatory standards while maintaining patient trust. A proactive approach to compliance will not only protect public health but also contribute to the long-term success and reputation of pharmaceutical organizations in an increasingly competitive landscape.
Similar Trending Products:
1. N-Nitroso Thiazolidine-4-Carboxylic Acid: https://aquigenbio.com/product/n-nitroso-thiazolidine-4-carboxylic-acid-2/
2. N-Nitroso Dabigatran: https://aquigenbio.com/product/n-nitroso-dabigatran/
3. 6-Amino-1-methyl-5-nitrosouracil: https://aquigenbio.com/product/6-amino-1-methyl-5-nitrosouracil-2/
Contact Us:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen Bio Sciences
Aquigen Bio Sciences is a leading contract research organization based in Pune, India, committed to providing advanced solutions for impurity standards and regulatory compliance in the pharmaceutical industry. Specializing in the analysis and management of nitroso impurities, including Solifenacin EP Impurity A Nitroso Impurity, Aquigen Bio Sciences is dedicated to improving safety and quality in drug development. By partnering with pharmaceutical manufacturers, Aquigen Bio Sciences navigates the regulatory landscape to ensure patient care remains the highest priority while addressing the challenges posed by nitroso contaminants.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Solifenacin EP Impurity A Nitroso Impurity: Navigating Compliance Risks, Regulatory Challenges, and Innovative Testing Solutions with Aquigen Bio Sciences to Safeguard Patient Safety and Uphold Pharmaceutical Standards here
News-ID: 3708989 • Views: …
More Releases from Aquigen Biosciences
Precision Standards for Oncology Research: Exploring Abemaciclib Impurity 1 and …
In the ever-evolving field of targeted cancer therapy, Abemaciclib has emerged as a pivotal agent in the treatment of hormone receptor-positive (HR+), HER2-negative advanced or metastatic breast cancer. As researchers and pharmaceutical developers continue to innovate in oncology, the importance of impurity profiling and the availability of reliable Abemaciclib impurity standards has never been greater.
At the forefront of pharmaceutical impurity standards, Aquigen Bio is proud to support global manufacturers, CROs,…
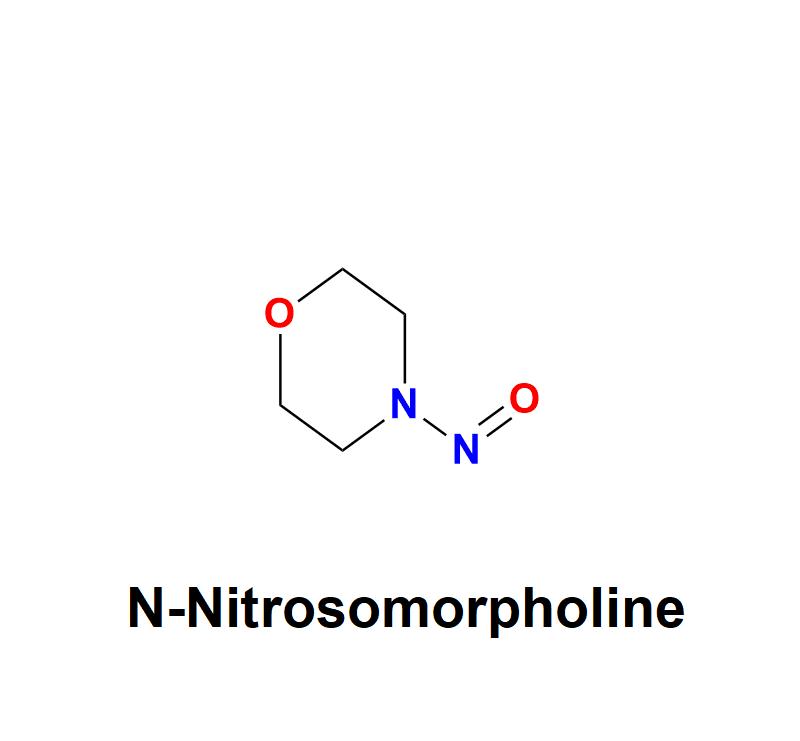
N-Nitrosomorpholine: Addressing Pharmaceutical Safety Challenges with Aquigen Bi …
N-Nitrosomorpholine, a compound belonging to the nitrosamine family, has garnered significant attention in the pharmaceutical and healthcare industries due to its potential carcinogenic risks. This chemical impurity, often found as a byproduct in manufacturing processes, poses serious challenges to drug safety and human health, necessitating stringent monitoring and control measures from pharmaceutical companies.
Learn more about N-Nitrosomorpholine: https://aquigenbio.com/product/n-nitrosomorpholine/
Understanding N-Nitrosomorpholine:
N-Nitrosomorpholine is a nitrosamine impurity characterized by its chemical structure, which includes…
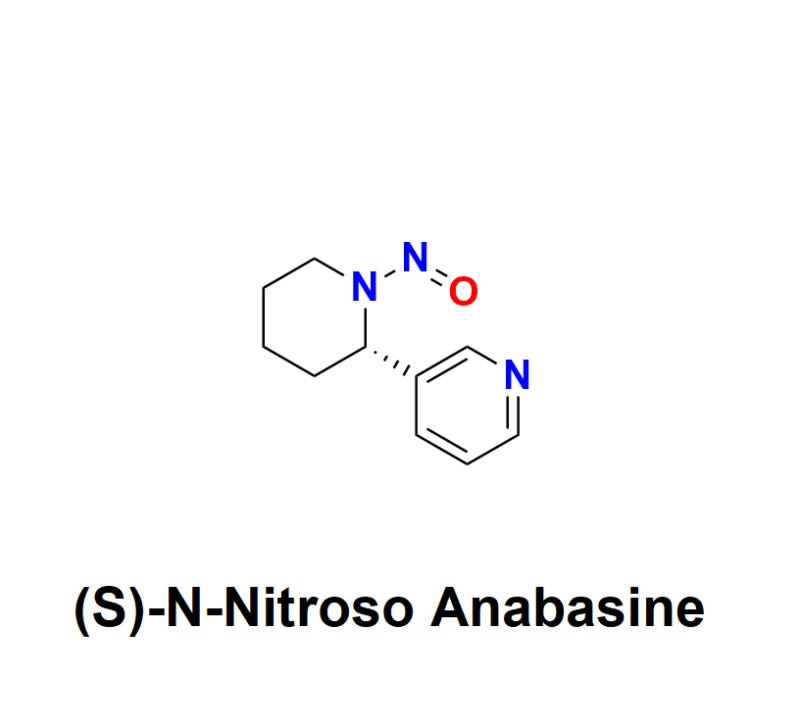
(S)-N-Nitroso Anabasine: Understanding the Risks, Regulatory Challenges, and How …
(S)-N-Nitroso Anabasine, a nitrosamine impurity, has raised significant safety concerns within the pharmaceutical industry. Recognized as a probable human carcinogen, this impurity has become a focal point for global regulatory agencies and manufacturers alike, urging a renewed emphasis on detection, prevention, and management.
Learn more about (S)-N-Nitroso Anabasine: https://aquigenbio.com/product/s-n-nitroso-anabasine/
What Is (S)-N-Nitroso Anabasine?
(S)-N-Nitroso Anabasine belongs to the family of nitrosamines, compounds formed through a chemical reaction known as nitrosation. This…
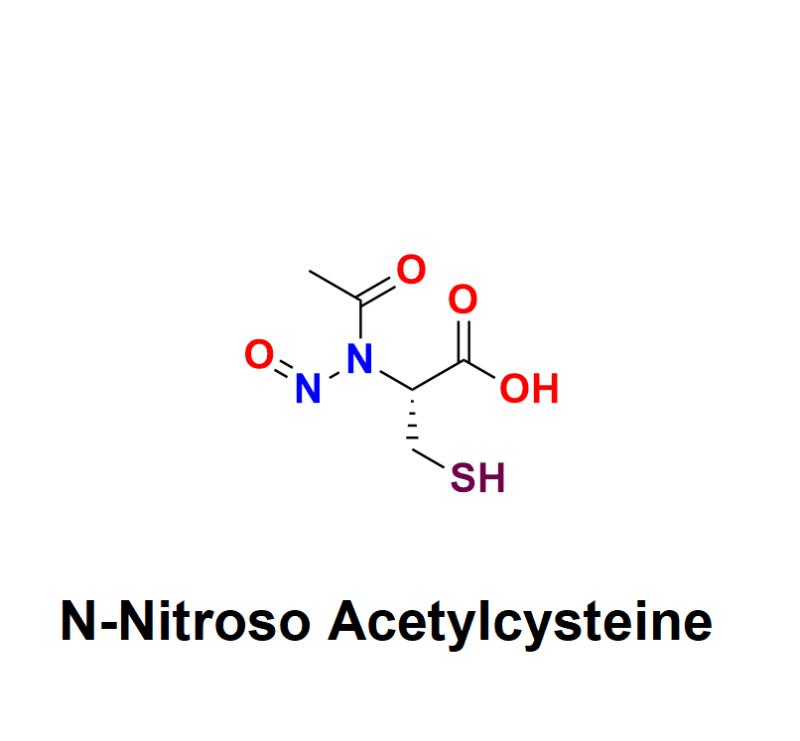
N-Nitroso Acetylcysteine: A Critical Concern in Pharmaceuticals - Exploring Haza …
N-Nitroso Acetylcysteine has emerged as a critical topic of concern in the pharmaceutical industry. As a member of the nitrosamine family, it is a potential impurity that poses significant health risks, including carcinogenicity, even in trace amounts. With increasing regulatory scrutiny on nitrosamine impurities, pharmaceutical manufacturers must address the presence of compounds like N-Nitroso Acetylcysteine to protect public health and ensure compliance with global standards.
Learn more about N-Nitroso Acetylcysteine: https://aquigenbio.com/product/n-nitroso-acetylcysteine/…
More Releases for Impurity
Tizanidine Nitroso Impurity 1 - High-Purity Reference Standard for Reliable Impu …
In the highly regulated world of pharmaceutical manufacturing, impurity profiling plays a critical role in ensuring drug safety, efficacy, and regulatory compliance. Among the wide range of impurity reference standards required during API development and validation, Tizanidine Nitroso Impurity 1 stands out as a vital compound for researchers and manufacturers working on Tizanidine-based formulations.
At Aquigen Bio, we are proud to offer Tizanidine Nitroso Impurity 1, a high-purity reference standard meticulously…
AquigenBio Launches High-Purity EP Impurity Standards for Propylthiouracil, Incl …
AquigenBio is pleased to announce the availability of two essential impurity reference standards-Propylthiouracil EP Impurity A and Propylthiouracil EP Impurity B. These impurity standards are now available to support pharmaceutical companies, contract research organizations, and analytical laboratories engaged in drug development, quality control, and regulatory compliance.
Propylthiouracil (PTU) is a well-known antithyroid medication, widely used in the treatment of hyperthyroidism. In line with pharmacopeial guidelines, it is critical to identify and…
Precision in Impurity Profiling: Aquigen Bio Introduces High-Purity Doxorubicin …
Pune, India - July 2025 - As regulatory standards tighten across the pharmaceutical industry, the need for accurate, high-purity reference standards has never been more critical. Aquigen Bio, a trusted provider of pharmaceutical reference materials, is proud to announce the availability of Doxorubicin Dimer Impurity 3, a key impurity used in the impurity profiling of the widely used chemotherapeutic agent, Doxorubicin.
This launch is part of Aquigen Bio's expanded portfolio of…
Breaking Ground in Pharmaceutical Impurity Standards: Aquigen Bio Expands Abirat …
In the evolving landscape of pharmaceutical development and quality control, the identification, synthesis, and supply of high-purity impurity standards play a pivotal role. One of the most significant advancements in recent times is the availability of specialized Abiraterone-related impurity standards-essential tools for characterizing drug formulations and ensuring regulatory compliance.
Manufactured and supplied by Aquigen BioSciences, a trusted leader in impurity synthesis and reference standards, these impurity standards offer analytical laboratories and…
Unlock Advanced Dabigatran Research with High-Purity Standards: Now Available - …
In the ever-evolving pharmaceutical landscape, precision, safety, and regulatory compliance have never been more crucial. With Dabigatran etexilate mesylate becoming a cornerstone in the management of thromboembolic disorders, the demand for high-purity standards and robust impurity profiling has surged. Recognizing this essential need, a new suite of advanced analytical tools for Dabigatran and its associated impurities has been made available to support the global scientific and quality control community.
To kno…
Aquigen Bio Expands Oncology Impurity Standards Portfolio with High-Purity Etopo …
India - 07/05/2025 - Aquigen Bio, a leading provider of pharmaceutical impurity reference standards, proudly announces the launch of its latest high-quality product: Etoposide EP Impurity A. This new addition to Aquigen's growing portfolio of oncology-related impurities is tailored to meet the stringent requirements of pharmaceutical manufacturers and analytical laboratories focused on drug safety, efficacy, and compliance.
To know more about : Etoposide EP Impurity A
https://aquigenbio.com/product/etoposide-ep-impurity-a/
A Strategic Addition to Oncology Drug…