Press release
AquigenBio Launches High-Purity EP Impurity Standards for Propylthiouracil, Including Impurity A (Thiourea) and Impurity B (Methylthiouracil), to Support Analytical Testing, Method Validation, and Regulatory Compliance
AquigenBio is pleased to announce the availability of two essential impurity reference standards-Propylthiouracil EP Impurity A and Propylthiouracil EP Impurity B. These impurity standards are now available to support pharmaceutical companies, contract research organizations, and analytical laboratories engaged in drug development, quality control, and regulatory compliance.Propylthiouracil (PTU) is a well-known antithyroid medication, widely used in the treatment of hyperthyroidism. In line with pharmacopeial guidelines, it is critical to identify and quantify impurities that may arise during its manufacturing or storage. The European Pharmacopoeia (EP) outlines the acceptable impurity profile for PTU, and AquigenBio's new impurity standards are specifically designed to meet those stringent requirements.
Propylthiouracil EP Impurity A - Thiourea
https://aquigenbio.com/product/propylthiouracil-ep-impurity-a/
Propylthiouracil EP Impurity A is chemically identified as thiourea, a known degradant and synthetic by-product associated with PTU. It is a small, sulfur-containing compound that must be monitored closely due to its potential biological activity and toxicological significance.
This impurity can be formed during the synthesis of propylthiouracil or during stability testing under certain environmental conditions such as heat, humidity, or oxidative stress. Regulatory authorities require its quantification and control during drug product development and production.
AquigenBio's reference standard for Impurity A is synthesized and purified under strict quality control systems. Each batch is supplied with a Certificate of Analysis (COA) and Safety Data Sheet (SDS), ensuring full traceability and compliance with international regulatory expectations. With this impurity standard, analytical chemists can confidently validate and execute impurity profiling methods such as HPLC, LC-MS, or UV-spectrophotometry for thiourea.
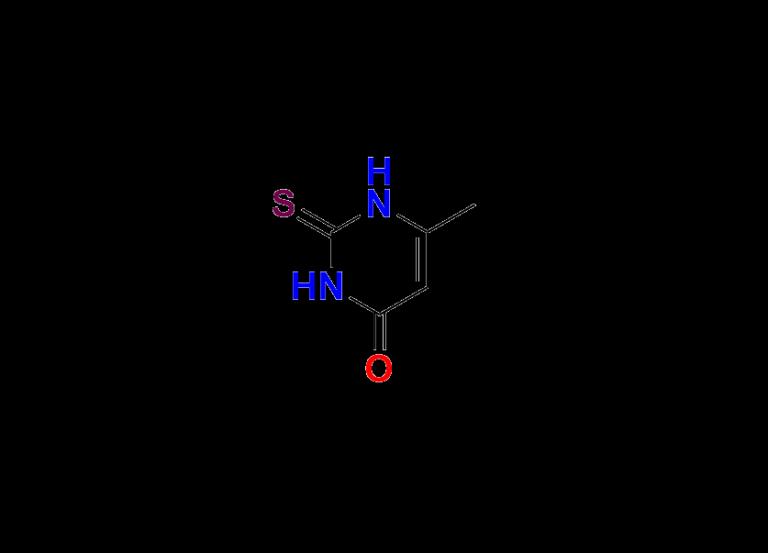
Propylthiouracil EP Impurity B - Methylthiouracil
https://aquigenbio.com/product/propylthiouracil-ep-impurity-b/
Propylthiouracil EP Impurity B, or methylthiouracil, is another critical secondary impurity associated with the propylthiouracil synthesis and degradation pathway. This compound is chemically more complex than thiourea and can appear in trace levels in active pharmaceutical ingredients (API) or formulated drug products.
Methylthiouracil is often detected under forced degradation studies and needs to be properly quantified in accordance with EP monographs. It may also be introduced during the synthetic process if reagents or reaction conditions are not tightly controlled.
AquigenBio's Impurity B standard is manufactured and qualified for use in analytical method development, system suitability testing, and regulatory submissions. It is provided in high-purity form, suitable for preparing calibration standards or spiking experiments in QC laboratories. This product plays an essential role in helping scientists ensure that their PTU products meet both internal quality standards and external regulatory thresholds.
Designed for Accuracy and Confidence
Both impurity standards are formulated for exclusive analytical use and are not intended for therapeutic or diagnostic applications. By providing these high-quality impurity reference standards, AquigenBio empowers pharmaceutical laboratories to carry out critical analytical functions, including:
Impurity profiling of raw materials, intermediates, and finished dosage forms
Method development and validation for analytical techniques such as HPLC and LC-MS
Stability studies and forced degradation tests
Routine quality control of production batches
Regulatory filings requiring full impurity characterization
These impurity standards are a key component of quality risk management and regulatory compliance for pharmaceutical companies working with propylthiouracil or related compounds.
The Role of Impurities in Drug Safety and Efficacy
In pharmaceutical science, impurities-whether process-related or degradation-related-can impact the safety, efficacy, and stability of drug products. Regulatory agencies such as the European Medicines Agency (EMA) and U.S. Food and Drug Administration (FDA) emphasize the need to monitor impurities and define clear limits based on toxicological data.
For propylthiouracil, impurities like thiourea and methylthiouracil must be monitored under ICH Q3A and Q3B guidelines. Even at low concentrations, such impurities may influence the pharmacological profile or cause unwanted side effects if left unquantified. Hence, their accurate identification and quantification are not just regulatory obligations-they are essential to ensuring patient safety and product reliability.
Explore Propylthiouracil Impurities
https://aquigenbio.com/products/impurity-standards/propylthiouracil/
By offering these EP-compliant standards, AquigenBio helps researchers and quality assurance teams maintain the highest level of product integrity.
Supporting Global Pharmaceutical Operations
AquigenBio's reference standards are available globally and can be rapidly dispatched to pharmaceutical companies, CROs, CMOs, academic institutions, and independent laboratories. With a strong commitment to quality, consistency, and scientific excellence, AquigenBio has earned the trust of professionals seeking reliable impurity standards for critical applications.
Each impurity standard undergoes rigorous analytical characterization and is accompanied by a detailed COA, including information on purity, identification, and handling. This ensures that every laboratory using AquigenBio's materials can do so with full confidence in the reliability of their analytical results.
Why Choose AquigenBio's Impurity Standards?
Pharmacopoeial alignment - Standards are prepared in accordance with EP monograph specifications
High-purity materials - Each batch is carefully synthesized and purified under controlled conditions
Traceable documentation - Includes COA, SDS, and batch-level data
Fast availability - Ready-to-ship inventory to meet urgent laboratory needs
Technical support - Scientific assistance available for method development and troubleshooting
AquigenBio works closely with research and development teams, analytical scientists, and regulatory affairs professionals to deliver impurity standards that facilitate smoother development cycles and greater confidence in analytical data.
Applications Across the Drug Lifecycle
These impurity standards are valuable at every stage of the pharmaceutical product lifecycle:
Preclinical and formulation development - For method validation and stress testing
Process optimization - To monitor potential synthetic by-products
Quality control - For batch release and routine QC
Stability testing - To track degradation over time
Regulatory submission - To support impurity specification in product dossiers
Their robust characterization and pharmacopeial compliance make them suitable for both R&D and GMP environments.
Contact:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About AquigenBio
AquigenBio is a trusted provider of pharmaceutical reference standards, specializing in impurity standards, working standards, and metabolite reference materials. With a commitment to scientific rigor and regulatory alignment, the company supports laboratories worldwide in producing safe, effective, and high-quality medicines.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release AquigenBio Launches High-Purity EP Impurity Standards for Propylthiouracil, Including Impurity A (Thiourea) and Impurity B (Methylthiouracil), to Support Analytical Testing, Method Validation, and Regulatory Compliance here
News-ID: 4128273 • Views: …
More Releases from Aquigen Bio Sciences

Elevate Pharmaceutical R&D with Aquigen BioSciences' Precision‐Grade Flibanser …
Flibanserin Impurity B is a reference standard used in pharmaceutical research and development. It is primarily applied during the analysis and validation of drug substances to identify, quantify, and control impurities that may be present in the final product. This impurity is associated with the parent compound, Flibanserin, a medication approved for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women.
Aquigen BioSciences offers Flibanserin Impurity B as a…

Estradiol Valerate EP Impurity A - Premium Reference Standard for Analytical Dev …
Estradiol Valerate EP Impurity A is a high-quality reference standard designed to meet the stringent requirements of pharmaceutical research, method validation, and quality control processes.
Explore Estradiol Valerate EP Impurity A :
https://aquigenbio.com/product/estradiol-valerate-ep-impurity-a/
Manufactured and characterized with precision, this impurity standard supports laboratories and manufacturers in achieving consistent, reliable, and reproducible results in critical analytical workflows.
With its exceptional purity and accurate characterization, Estradiol Valerate EP Impurity A plays a vital role…

High-Purity N-Nitroso Betahistine D3 for Precise Pharmaceutical Analysis | Deute …
Product Overview
N-Nitroso Betahistine D3 is a premium deuterated nitrosamine impurity standard, specifically developed for precise analytical testing in pharmaceutical laboratories. This reference standard is widely used for analytical method development, validation, and quality control processes to meet stringent regulatory guidelines. With exceptional purity, complete documentation, and reliable traceability, it is ideal for research, development, and compliance applications.
https://aquigenbio.com/product/n-nitroso-betahistine-d3/
Key Features and Benefits
Deuterated Design for Precision: The incorporation of deuterium improves mass spectrometric…

Aquigen Bio Strengthens Pharmaceutical Research with High-Purity Icatibant Impur …
Aquigen Bio, a trusted supplier of pharmaceutical reference standards, today announced the expansion of its Icatibant Impurity Standards portfolio, designed to support drug developers, analytical laboratories, and research organizations with reliable materials for impurity profiling and quality control.
Icatibant, a selective bradykinin B2 receptor antagonist, is widely used in the treatment of hereditary angioedema (HAE). Given its peptide-based structure, Icatibant is prone to the formation of impurities during synthesis and storage.…
More Releases for AquigenBio
AquigenBio Introduces High-Purity Daridorexant Hydrochloride to Support Sleep Di …
India - August 2025 - AquigenBio, a premier provider of pharmaceutical reference standards and impurity profiling solutions, proudly announces the release of Daridorexant Hydrochloride (CAS No. 1792993-84-0). This high-quality, precisely characterized compound is a hydrochloride salt form of Daridorexant, a dual orexin receptor antagonist with proven efficacy in the treatment of insomnia.
Explore Daridorexant Hydrochloride -
https://aquigenbio.com/product/daridorexant-hydrochloride/
The launch of this product marks a significant addition to AquigenBio's growing portfolio of sleep…
Precision in Pharmaceutical Analysis: Resmetirom Impurity Standards from Aquigen …
Resmetirom is a breakthrough thyroid hormone receptor-β agonist, currently under investigation for the treatment of nonalcoholic steatohepatitis (NASH). The successful development, validation, and filing of Resmetirom-based therapies require precision in impurity profiling - a critical cornerstone of global regulatory acceptance.
Why Choose AquigenBio's Resmetirom Impurities?
1. High-Purity Reference Standards: Each impurity is synthesized and purified to >95% purity, with robust documentation including HPLC, MS, and NMR characterization data for your quality assurance.
2.…
AquigenBio Expands Ibuprofen Impurity Standards Portfolio for Advanced Pharmaceu …
AquigenBio, a trusted global provider of high-quality impurity standards and reference materials, has announced the expansion of its Ibuprofen Impurity Standards portfolio. These new offerings are designed to meet the growing demand for precise analytical tools in pharmaceutical research, quality control, and regulatory compliance.
The updated category now includes specialized impurity standards for Ibuprofen and its related compounds, delivering high-purity reference materials that meet stringent industry and regulatory requirements.
Newly Featured Ibuprofen…
Aquigen Bio Introduces N‐Nitroso Pirfenidone EP Impurity B - A High-Purity Ref …
Pune-/Delran- Aquigen Bio is pleased to announce the introduction of N‐Nitroso Pirfenidone EP Impurity B, a premium reference standard designed for the highest rigor in pharmaceutical analysis and regulatory compliance. Available via custom‐synthesis, this product serves as an essential resource for analytical method development (AMD), method validation (AMV), quality control (QC), and support for Abbreviated New Drug Applications (ANDA)
https://aquigenbio.com/product/n-nitroso-pirfenidone-ep-impurity-b/
Product Highlights
Name: N‐Nitroso Pirfenidone EP Impurity B
Catalog No: AQ‐N000968
CAS No: 2512237‐43‐1
Molecular…
Unveiling Tegoprazan Impurity 2: A Critical Reference Standard in P-CAB Drug Dev …
As the pharmaceutical industry advances toward next-generation acid suppression therapies, potassium-competitive acid blockers (P-CABs) like Tegoprazan are emerging as game changers in the treatment of gastroesophageal reflux disease (GERD) and other acid-related conditions. However, to ensure the safety, efficacy, and regulatory compliance of P-CAB-based drugs, identifying and monitoring impurities during synthesis and storage is non-negotiable.
Explore Tegoprazan Category
https://aquigenbio.com/products/impurity-standards/tegoprazan/
One such critical impurity is Tegoprazan Impurity 2 - a process-related compound now available…
Exploring Key Dabigatran Derivatives: A Closer Look at N-Methoxycarbonyl and O-A …
Dabigatran is a well-established direct thrombin inhibitor used primarily in anticoagulation therapy to prevent stroke and treat deep vein thrombosis or pulmonary embolism. As with many active pharmaceutical ingredients (APIs), the analysis and synthesis of its related substances-particularly esterified and modified forms-play a crucial role in pharmaceutical research, formulation development, and regulatory compliance.
This article delves into four important derivatives of Dabigatran:
N-Methoxycarbonyl Dabigatran Ethyl Ester
O-(3-Hexyl) Dabigatran Ethyl Ester
O-Butyl Dabigatran Ethyl Ester
O-Desethyl…