Press release
Breaking Ground in Pharmaceutical Impurity Standards: Aquigen Bio Expands Abiraterone Impurity Portfolio for Advanced Research Applications
In the evolving landscape of pharmaceutical development and quality control, the identification, synthesis, and supply of high-purity impurity standards play a pivotal role. One of the most significant advancements in recent times is the availability of specialized Abiraterone-related impurity standards-essential tools for characterizing drug formulations and ensuring regulatory compliance.Manufactured and supplied by Aquigen BioSciences, a trusted leader in impurity synthesis and reference standards, these impurity standards offer analytical laboratories and pharmaceutical R&D teams the resources they need to enhance the safety, efficacy, and stability of steroidal antiandrogens such as Abiraterone.
To know more about Abirateron Impurities
https://aquigenbio.com/products/impurity-standards/abiraterone/
The Role of Impurity Profiling in Modern Drug Testing
Abiraterone Acetate is a potent CYP17 inhibitor widely used in the treatment of metastatic castration-resistant prostate cancer (mCRPC). Like all active pharmaceutical ingredients (APIs), Abiraterone undergoes degradation and transformation during synthesis, formulation, and storage-leading to the formation of related substances and impurities.
One of the key reference standards now available from Aquigen Bio is linked here:
This compound is critical for:
Stability studies
Impurity profiling
Method development and validation
Regulatory submissions to global health authorities (FDA, EMA, CDSCO)
As regulatory bodies emphasize the identification and quantification of all potential impurities beyond specified thresholds (typically 0.05-0.1%), the presence of a certified reference standard becomes indispensable.
Unmatched Purity and Characterization by Aquigen Bio
Aquigen BioSciences has taken the lead in synthesizing and characterizing several Abiraterone-related impurities to the highest analytical standards. These reference standards are:
Supplied with full Certificate of Analysis (CoA)
Backed by NMR, Mass Spectrometry, IR, and HPLC purity data
98% pure, suitable for regulatory and analytical use
This level of detail ensures reproducibility, reliability, and consistency in pharmaceutical quality assurance programs.
Connecting the Dots: Abiraterone and Its Impurity Pathways
Understanding the metabolic and synthetic degradation pathways of Abiraterone is essential for developing a complete impurity profile. Several structurally related impurities can form during its synthesis and storage, including:
1. 3-Deoxy-3-Chloroabiraterone
https://aquigenbio.com/product/3-deoxy-3-chloroabiraterone/
This halogenated impurity may arise during chlorination steps or from residual chlorinated intermediates. It signifies the need for strict control of chlorinating agents and purification steps.
2. 3-Deoxy-3-Acetyl Abiraterone-3-Ene
https://aquigenbio.com/product/3-deoxy-3-acetyl-abiraterone-3-ene/
An acetylated variant that appears as a result of esterification or incomplete hydrolysis reactions. Monitoring its presence helps assess the effectiveness of synthesis and purification protocols.
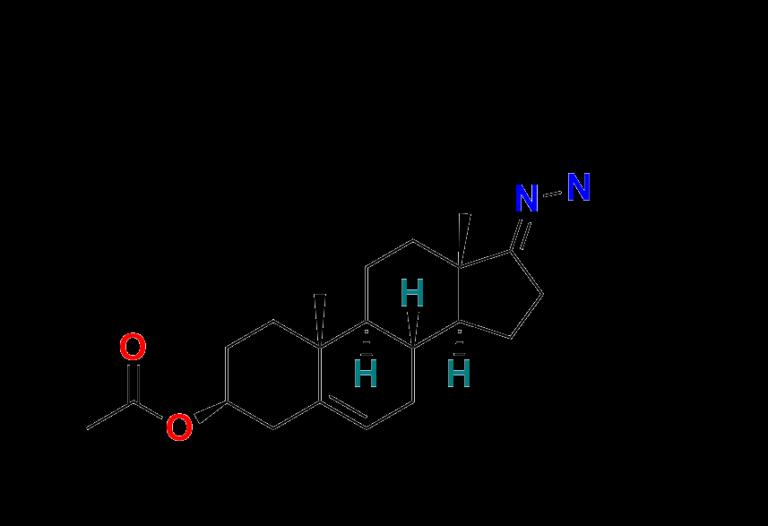
3. 3-O-Acetylandrostenone Hydrazone
https://aquigenbio.com/product/3-o-acetylandrostenone-hydrazone/
A more complex impurity, often found in stress and forced degradation studies. It can provide insights into the molecule's behavior under oxidative or reductive environments.
The availability of these impurities, alongside other reference standards, allows pharmaceutical teams to conduct in-depth quality and stability testing.
Regulatory Importance: ICH Guidelines and Impurity Standards
According to ICH Q3A and Q3B guidelines, all identified impurities above the qualification threshold must be:
Chemically characterized
Quantified using validated analytical methods
Justified through toxicological data or specifications
Failure to characterize and control impurities can result in compliance issues, delayed approvals, or even product recalls. This highlights the need for high-quality, traceable impurity standards from reliable sources like Aquigen BioSciences.
Global Availability with Aquigen BioSciences
Aquigen Bio's global logistics and supply network ensures that pharmaceutical companies, CROs, and research labs can access these impurity standards with minimal lead times.
Highlights include:
Worldwide shipping with secure and temperature-controlled packaging
Batch consistency for multi-site studies
Custom synthesis services for unique or rare impurities
Comprehensive regulatory documentation for global market submissions
Why Choose Aquigen Bio for Abiraterone-Related Impurities?
Aquigen BioSciences is a globally trusted supplier of pharmaceutical reference standards. Here's what makes them stand out:
Specialized expertise in steroidal impurity chemistry
High-end analytical and spectroscopic facilities
Tailored custom synthesis for niche research
Strict documentation, CoA, and spectral validation
Whether you're conducting early-phase research or working toward regulatory submission, Aquigen offers the tools and expertise to help you succeed.
Sustainability, Innovation, and Integrity
Aquigen Bio is also committed to green chemistry principles, including the use of environmentally safe reagents, reduced solvent waste, and responsible production methods. The company operates in accordance with ISO 9001:2015 guidelines and maintains GMP-inspired practices.
Contact:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen BioSciences
Aquigen BioSciences is a premier manufacturer of impurity reference standards, stable isotope compounds, and custom organic molecules for global pharmaceutical, biotech, and research markets. Headquartered in India and serving customers worldwide, Aquigen brings chemistry excellence to every molecule.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Breaking Ground in Pharmaceutical Impurity Standards: Aquigen Bio Expands Abiraterone Impurity Portfolio for Advanced Research Applications here
News-ID: 4053401 • Views: …
More Releases from Aquigen Bio Sciences

Elevate Pharmaceutical R&D with Aquigen BioSciences' Precision‐Grade Flibanser …
Flibanserin Impurity B is a reference standard used in pharmaceutical research and development. It is primarily applied during the analysis and validation of drug substances to identify, quantify, and control impurities that may be present in the final product. This impurity is associated with the parent compound, Flibanserin, a medication approved for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women.
Aquigen BioSciences offers Flibanserin Impurity B as a…

Estradiol Valerate EP Impurity A - Premium Reference Standard for Analytical Dev …
Estradiol Valerate EP Impurity A is a high-quality reference standard designed to meet the stringent requirements of pharmaceutical research, method validation, and quality control processes.
Explore Estradiol Valerate EP Impurity A :
https://aquigenbio.com/product/estradiol-valerate-ep-impurity-a/
Manufactured and characterized with precision, this impurity standard supports laboratories and manufacturers in achieving consistent, reliable, and reproducible results in critical analytical workflows.
With its exceptional purity and accurate characterization, Estradiol Valerate EP Impurity A plays a vital role…

High-Purity N-Nitroso Betahistine D3 for Precise Pharmaceutical Analysis | Deute …
Product Overview
N-Nitroso Betahistine D3 is a premium deuterated nitrosamine impurity standard, specifically developed for precise analytical testing in pharmaceutical laboratories. This reference standard is widely used for analytical method development, validation, and quality control processes to meet stringent regulatory guidelines. With exceptional purity, complete documentation, and reliable traceability, it is ideal for research, development, and compliance applications.
https://aquigenbio.com/product/n-nitroso-betahistine-d3/
Key Features and Benefits
Deuterated Design for Precision: The incorporation of deuterium improves mass spectrometric…

Aquigen Bio Strengthens Pharmaceutical Research with High-Purity Icatibant Impur …
Aquigen Bio, a trusted supplier of pharmaceutical reference standards, today announced the expansion of its Icatibant Impurity Standards portfolio, designed to support drug developers, analytical laboratories, and research organizations with reliable materials for impurity profiling and quality control.
Icatibant, a selective bradykinin B2 receptor antagonist, is widely used in the treatment of hereditary angioedema (HAE). Given its peptide-based structure, Icatibant is prone to the formation of impurities during synthesis and storage.…