Press release
Lipid Nanoparticles, Key Component of COVID-19 mRNA Vaccines
Lipid nanoparticles have been developed as vehicles for small molecule delivery by the nanomedicine and materials communities and are now a key component of COVID-19 mRNA vaccines.Vaccines against COVID-19 have been developed with unprecedented speed. In particular, mRNA vaccines — a technology already in clinical trials for other infectious diseases, such as influenza — have shown impressive efficacy in clinical trials.
mRNA vaccines rely on the delivery of mRNA into the cytoplasm of host cells, where it can be transcribed into antigenic proteins to trigger the production of neutralizing antibodies. However, mRNA is three to four orders of magnitude larger than molecules that readily diffuse into cells; in addition, the dense negative charge of mRNA electrostatically repulses the anionic cell membrane, preventing its uptake. Therefore, mRNA vaccines require a delivery vehicle that not only protects the nucleic acid from degradation but allows the mRNA to get into cells.
BioNTech/Pfizer’s and Moderna’s mRNA vaccines both use lipid nanoparticles as mRNA carriers. The impressive speed at which these vaccines could be developed is partly owed to the fact that nucleic acid delivery by lipid nanoparticles has long been investigated and optimized by the nanomedicine community, who thoroughly studied lipid nanoparticle chemistry, structure, surface, injection routes, uptake, endosomal escape, cargo release, dosage, clearance and, importantly, safety.
Owing to their size and properties, lipid nanoparticles are taken up by cells via endocytosis, and the ionizability of the lipids at low pH (likely) enables endosomal escape, which allows release of the cargo into the cytoplasm. In addition, lipid nanoparticles usually contain a helper lipid to promote cell binding, cholesterol to fill the gaps between the lipids, and a polyethylene glycol (PEG) to reduce opsonization by serum proteins and reticuloendothelial clearance. The relative amounts of ionizable lipid, helper lipid, cholesterol and PEG substantially affect the efficacy of lipid nanoparticles, and need to be optimized for a given application and administration route.
It was a long road to optimizing lipid nanoparticle formulations for nucleic acid delivery. To achieve clinical efficacy, every aspect of the lipid nanoparticle formulation had to be optimized, and more than 300 ionizable lipids had to be screened. Furthermore, all key steps of the lipid nanoparticle journey in the body had to be understood before clinical trials could begin. This knowledge has certainly contributed to the rapid development of COVID-19 mRNA vaccines.
From a materials science perspective, the success of lipid nanoparticle mRNA vaccines is exciting and important, as it underlines the value of materials science for medical advances and motivates further fundamental and applied nanoparticle research, which will hopefully be reflected in future funding cycles.
Huateng Pharma, a professional PEG supplier, is dedicated to manufacturing and supplying a wide range of PEG derivatives. Lipid nanoparticles is a key component of COVID-19 mRNA vaccines. We can supply below PEG products which are excipients used in COVID-2019 vaccine.
1.ALC-0159, mPEG-N,N-Ditetradecylacetamide, CAS No. 1849616-42-7
2.mPEG-DMG, CAS NO. 160743-62-4
3.mPEG-CH2CH2CH2-NH2
4.mPEG-CM
5.mPEG-OH, CAS NO. 9004-74-4
Hunan Huateng Pharmaceutical Co. Ltd.
Telephone: +86 731 89916275
Fax: +86 0731-82251112-818
Website: https://en.huatengsci.com
Email: sales@huatengusa.com
Human Huateng Pharmaceutical Co., Ltd. is a leading and professional manufacturer which can provide pharmaceutical intermediates, PEG derivatives, biochemical reagents, APIs, Vitamin D Derivatives and so on.
Huateng Pharma is committed to providing cost-effective pharmaceutical products and services to customers around the world with the belief of “Strive for the Human Health".
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Lipid Nanoparticles, Key Component of COVID-19 mRNA Vaccines here
News-ID: 2299103 • Views: …
More Releases from Hunan Huateng Pharmaceutical Co. Ltd.

Huateng Pharma Supplies Minoxidil Intermediate 2,4-Diamino-6-chloropyrimidine (C …
Minoxidil was first introduced by Upjohn Company of the United States, and was first used as an oral drug for the treatment of refractory hypertension in the 1970s. In later clinical applications, doctors observed hair regrowth and generalized excessive hair in balding patients, which led to the development of minoxidil preparations.
Minoxidil can increase local blood supply, stimulate the proliferation and differentiation of hair follicle epithelial cells, so as to promote…

Huateng Pharma Supplies Anti-diabetic API Intermediates With Huge Stock
Diabetes is a serious chronic disease characterized by elevated blood sugar concentrations associated with the effects of abnormal beta cell biology on insulin action. Diabetes occurs when the pancreas does not produce enough insulin or the body does not use the insulin it does produce efficiently.
The most common forms of diabetes are type 1 diabetes and type 2 diabetes. Type 1 diabetes is characterized by insufficient insulin production and…
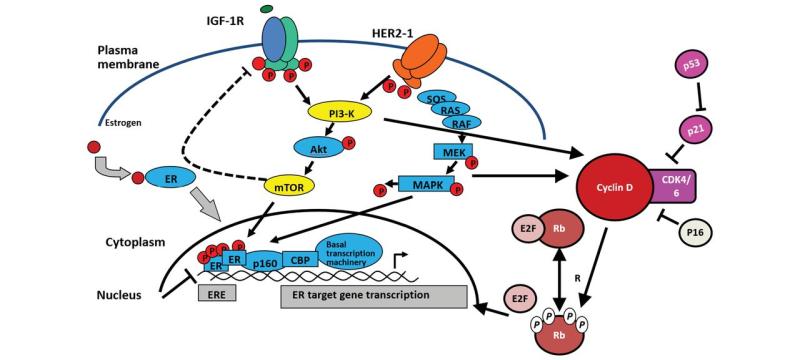
Huateng Pharma Supplies Some Intermediates of CDK4/6 Inhibitors for Treatment of …
Breast cancer is the most frequent cancer among women, impacting 2.1 million women each year, and also causes the greatest number of cancer-related deaths among women. In 2018, it is estimated that 627,000 women died from breast cancer - that is approximately 15% of all cancer deaths among women. While breast cancer rates are higher among women in more developed regions, rates are increasing in nearly every region globally.
The majority…
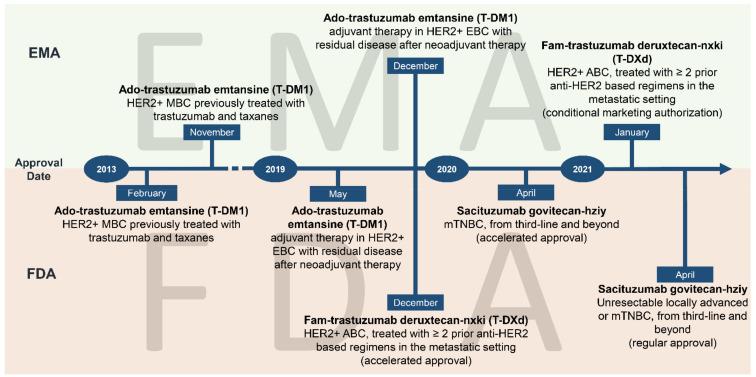
ADC Drugs For HER2 Positive Breast Cancer
According to the latest data, breast cancer has overtaken lung cancer to become the most common cancer among women, and the death rate is the second highest among female tumors, seriously affecting the physical and mental health of women around the world. Patients with abnormal expression of human epidermal growth factor receptor (HER2) account for 15%-20% of all breast cancers, which is highly invasive and has poor prognosis.
Although more drug…
More Releases for PEG
Biopharma PEG Delivers Innovative PEG Solutions for Enhanced Drug Efficacy
Watertown, MA - October 24, 2024 - Biopharma PEG is excited to announce its extensive offerings of polyethylene glycol (PEG) products, including monofunctional, homobifunctional, heterobifunctional, and multi-arm PEGs, tailored for PEGylation in biopharmaceutical applications. With over 40 PEGylated drugs approved globally, the role of PEGylation in drug development is more critical than ever.
PEGylation provides numerous advantages, such as improved solubility, enhanced stability, and increased circulation time in the bloodstream. By…
Biopharma PEG Expands Multi-Arm PEG Product Line
Biopharma PEG, a leader in PEG derivatives, is excited to announce the expansion of its high-purity Multi-Arm PEG linker product line, catering to the evolving needs of the medical and bioorganic fields. These advanced PEG linkers are available in various functional groups and molecular weights ranging from 1k to 40k, offering unmatched versatility and performance for research and development in cutting-edge medical applications.
"Biopharma PEG is committed to delivering high-purity multi-arm…
Biopharma PEG Supplies PEG Products Used For Infectious Disease Vaccines
As of January 1, 2023, global vaccine development includes a total of 966 vaccine candidates, of which 23% (220) are traditional inactivated or attenuated vaccines. Advances in molecular technology have facilitated the development of other platforms, such as recombinant protein vaccines, nucleic acid vaccines, and viral vector vaccines, which have further diversified global vaccine development.
Recombinant protein vaccines accounted for the largest proportion of all pipeline in development, 22% (215), due…
Biopharma PEG Supplies PEG Products For Click Chemistry Reactions
What is "click chemistry"? "Click Chemistry", this is a literary name given to this kind of reaction by the Nobel Prize winner K.Burry Sharless, when the cards are put together, "click" (click). Simply put, it is to add two structures to two molecules respectively, and these two structures can be specifically combined to synthesize the required chemical molecules. One of the most famous click-chemistry reactions is the Cu-catalyzed azide-alkyne cycloaddition…
Biopharma PEG Develops PEG Linkers for Antibody Drug Conjugates
Antibody-drug conjugate (ADC) is one of the fastest growing fields in tumor therapy, which consists of monoclonal antibody (Antibody), linker (Linker) and active drug (Payload). So far, there are only 15 drugs on the market in the world. However, with the development of some perfect antibody modification techniques, advanced site-specific coupling techniques and powerful small-molecule toxins, ADC drug research has mushroomed and a large number of ADC drugs are in…
Biopharma PEG Provides Multi-arm PEG Derivatives Crosslinked Into Hydrogels
Polyethylene (ethylene glycol) is a hydrophilic polymer that can have a very high water content when cross-linked into a network. Polyethylene glycol (PEG) is a suitable material for biological applications because it does not normally elicit an immune response. Since the 1970s, PEG has been used to modify therapeutic proteins and peptides in order to increase their solubility, reduce their toxicity, and prolong their cyclic half-lives. In the late 1970s,…