Press release
ADC Drugs For HER2 Positive Breast Cancer
According to the latest data, breast cancer has overtaken lung cancer to become the most common cancer among women, and the death rate is the second highest among female tumors, seriously affecting the physical and mental health of women around the world. Patients with abnormal expression of human epidermal growth factor receptor (HER2) account for 15%-20% of all breast cancers, which is highly invasive and has poor prognosis.Although more drug choices such as pertuzumab and lapatinib on the basis of trastuzumab significantly prolong the survival of patients and further improve the prognosis, many patients are not sensitive to treatment and relapse and drug resistance occur in a short time, and the efficacy of traditional HER2-targeted drugs for breast cancer patients with low HER2 expression is not optimistic.
Mechanism of action of ADC drugs
Antibody conjugate drugs (ADCs) are a type of novel antitumor drugs in continuous development. They combine the high specificity of monoclonal antibody drugs with the high activity of small molecule cytotoxic drugs, which can improve the targeting of antitumor drugs and reduce the probability of toxic and side effects.
An antibody drug conjugate (ADC) consists of three core elements: an antibody that targets a target antigen, a cytotoxic payload, and a linker that binds the two.
Potent cytotoxic drugs can be targeted to tumor cells with chemotherapeutic cytotoxic properties by means of selective monoclonal antibodies against specific or preferentially expressed antigens on target cells. Free cytotoxic agents can be used to create a more favorable therapeutic window for cytotoxic drugs, which not only reduces the concentration of peripheral toxic drugs, but also improves the efficiency and intensity of effective anti-tumor substances. Some ADCs show efficacy by targeting delivery payloads in breast cancers with low HER2 expression.
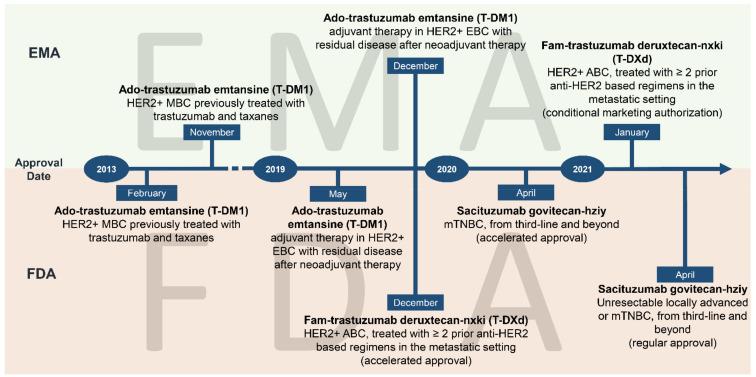
Of the 15 ADC drugs currently approved, three are used to treat breast cancer: Enhertu (T-DXd, DS-8201), Trodelvy (sacituzumab govitecan/SG, Goxaltuzumab) and ado-trastuzumab emtansine (T-DM1). Two are for HER2 breast cancer ADCs.
T-DM1(Ado-trastuzumab Emtansine)
T-DM1 is the first ADC drug to target HER2-positive breast cancer.
T-DM1, which is bound by trastuzumab and tubulin inhibitor DM1 via a stable, non-lytic, non-reducing thioether linker, was first approved by the FDA in 2013 for use in advanced breast cancer patients with HER2-positive metastases or who have been treated with trastuzumab and taxanes drugs.
Its approval was based on the EMILIA Phase III clinical study and the EMILIA Bridging Study in China, which showed that T-DM1 improved overall survival (OS) and progression-free survival (PFS), as well as better objective response rate (ORR) and median duration of response (DoR) compared to Lapatinib combined with capecitabine.
It will be listed in China in 2020, it has been approved for adjuvant therapy in HER2-positive early breast cancer patients with residual invasive lesions (non-pCR) after receiving taxane combined trastuzuab-based neoadjuvant therapy and for monotherapy in HER2-positive, unresectable locally advanced or metastatic breast cancer patients receiving taxane combined trastuzumab therapy.
Therefore, in the 2023 update of CSCO Guidelines for the Diagnosis and treatment of breast cancer, for patients with HER-2 positive breast cancer after neoadjuvant therapy, only trastuzumab was used in preoperative anti-HER-2 treatment, and non-pCR, T-DM1 evidence recommended for grade I was adjusted from 1B to 1A. For patients with HP and non-pCR in preoperative anti-HER-2 therapy, the evidence for T-DM1 recommended for grade I was adjusted from 1B to 2A. In addition, in the rescue treatment of advanced breast cancer with positive HER-2, the evidence level of "T-DM1" in trastuzumab treatment failure stratified patients was adjusted from 1B to 1A.
At present, there are also clinical trials on T-DM1 in CompassHER2 Rd test to evaluate whether the compasTI SHER2 test in combination with the tyrosine kinase inhibitor Tucatinib is better than T-DM1 alone. The HER2CLIMB-02 trial evaluated whether combination with Tucatinib was superior to T-DM1 monotherapy in patients previously receiving taxus + trastuzumab.
T-DXd, DS-8201 (Trastuzumab)
T-DXd (DS-8201) is an ADC that combines the anti-HER2 monoclonal antibody Trastuzumab with a topoisomerase Ⅰ inhibitor (Dxd) by means of an enzyme-cleavable tetrapeptidyl ligand.
Launched in the United States on December 21, 2019, the drug is the first official FDA approval of trastuzumab for the posterior treatment of HER2-positive breast cancer, based on stunning data from the DESTINY Breast01 study. Earlier this year, trastuzumab was approved domestically as a single drug for the treatment of unresectable or metastatic HER2-positive adults with breast cancer who have previously received one or more anti-HER2 drugs.
Up to now, trastuzumab has been approved in at least 5 tumor indications worldwide, including HER2-positive breast cancer, gastric cancer, gastroesophageal junction cancer, breast cancer with low HER2 expression, and non-small cell lung cancer.
Its domestic approval is based primarily on DESTINY Breast03, which has shown that trastuzumab (T-DXd), in contrast to Ado-trastuzumab Emtansine (T-DM1), is effective in treating HER2-positive unresectable and/or metastatic breast cancer in patients previously treated with trastuzumab and taxane. Reduced the risk of disease progression or death by 72% (hazard ratio [HR] 0.28; 95% confidence interval [CI] 0.22-0.37; p
Hunan Huateng Pharmaceutical Co. Ltd.
Address: Lugu Business Plaza E1, Yuelu District, Changsha City, Hunan Province, China
Manufacturing Base: Tongguan Kiln, Wangcheng district, Changsha, Hunan, China
Email: sales@huatengusa.com
Website: https://us.huatengsci.com
Zip code: 410205
Telephone: +86 731 89916275
Fax: +86 0731-82251112-818
Huateng Pharma is a leading and professional manufacturer which can provide pharmaceutical intermediates, PEG derivatives, biochemical reagents, APIs, Vitamin D Derivatives and so on. We have a 34,000 square meter manufacturing site with advanced design concept of intelligent manufacturing, which can realize the integration of the production process and accomplish the complete transformation of lab scale - pilot plant - large-scale commercial production, with an annual capacity of more than 1 billion RMB.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release ADC Drugs For HER2 Positive Breast Cancer here
News-ID: 3063323 • Views: …
More Releases from Hunan Huateng Pharmaceutical Co. Ltd.

Huateng Pharma Supplies Minoxidil Intermediate 2,4-Diamino-6-chloropyrimidine (C …
Minoxidil was first introduced by Upjohn Company of the United States, and was first used as an oral drug for the treatment of refractory hypertension in the 1970s. In later clinical applications, doctors observed hair regrowth and generalized excessive hair in balding patients, which led to the development of minoxidil preparations.
Minoxidil can increase local blood supply, stimulate the proliferation and differentiation of hair follicle epithelial cells, so as to promote…

Huateng Pharma Supplies Anti-diabetic API Intermediates With Huge Stock
Diabetes is a serious chronic disease characterized by elevated blood sugar concentrations associated with the effects of abnormal beta cell biology on insulin action. Diabetes occurs when the pancreas does not produce enough insulin or the body does not use the insulin it does produce efficiently.
The most common forms of diabetes are type 1 diabetes and type 2 diabetes. Type 1 diabetes is characterized by insufficient insulin production and…
Huateng Pharma Supplies Some Intermediates of CDK4/6 Inhibitors for Treatment of …
Breast cancer is the most frequent cancer among women, impacting 2.1 million women each year, and also causes the greatest number of cancer-related deaths among women. In 2018, it is estimated that 627,000 women died from breast cancer - that is approximately 15% of all cancer deaths among women. While breast cancer rates are higher among women in more developed regions, rates are increasing in nearly every region globally.
The majority…

TPD Show Potential For The Treatment of Alzheimer's Disease
Targeted protein degradation (TPD) is a promising strategy in the field of drug discovery. In recent years, targeted protein degradation (TPD) technology has developed rapidly, especially proteolysis targeting chimera (PROTAC), which is the most representative technology of TPD strategy. TPD drugs are one of the hot spots of new drug development in recent years, especially in the field of oncology. For example, a TPD drug developed by Arvinas has achieved…
More Releases for HER2
HER2-Positive Breast Cancer (HER2+ BC) Clinical Market to Reach USD 21.46 Billio …
Sub-Headline: The global HER2-Positive Breast Cancer Clinical Market is expected to rise from USD 13.82 billion in 2023 to USD 21.46 billion by 2030, registering a CAGR of 6.5%, driven by rapid uptake of antibody-drug conjugates (ADCs), dual-targeted therapies, and AI-enabled precision oncology diagnostics.
Introduction
The HER2-Positive Breast Cancer (HER2+ BC) Clinical Market is undergoing a major transformation fueled by next-generation targeted therapies, breakthrough ADCs, biosimilar expansion, and genomic testing advancements. HER2+…
Evolving Market Trends In The HER2-Positive Breast Cancer Industry: Innovative T …
Our market reports now include the latest updates on global tariffs, trade impacts, and evolving supply chain dynamics.
What Is the Expected HER2-Positive Breast Cancer Market Size During the Forecast Period?
Over the past few years, there has been a noteworthy expansion in the HER2-positive breast cancer market. The market, which is projected to increase from $10.21 billion in 2024 to $10.96 billion in the following year, predicts a compound annual growth…
HER2 Inhibitors: Advancing Breast Cancer Treatments
"The Business Research Company recently released a comprehensive report on the Global HER2 Inhibitors Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive Sample…
HER2 Positive Breast Cancer Pipeline Drugs 2024
DelveInsight's, "HER2 Positive Breast Cancer Pipeline Insight 2024" report provides comprehensive insights about 60+ companies and 65+ pipeline drugs in HER2 Positive Breast Cancer pipeline landscape. It covers the HER2 Positive Breast Cancer pipeline drug profiles, including clinical and nonclinical stage products. It also covers the HER2 Positive Breast Cancer therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in…
HER2 Inhibitors: Targeted Therapies Transforming Cancer Treatment
HER2 inhibitors market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses the market to account to grow at a CAGR of 9% in the above mentioned forecast period.
Global HER2 Inhibitors Market Scope and Market Size
The HER2 inhibitors market is segmented on the basis of treatment, application, end-users and distribution channel. The growth amongst these segments will help you analyze…
Her2 Antibodies Market: Industry Overview and Key Factors
Global Her2 Antibodies Market: Overview
Human epidermal growth factor receptor 2 (Her2) refers to an oncogene whose over-expression or amplification is commonly associated with the development of an aggressive type of breast cancer. Patients having the over-expression of Her2 receptors are diagnosed with the help of tests such as Fluorescent In-Situ Hybridization (FISH) and Immunohistochemistry (IHC).
The American Cancer Society states that around 15-30% of breast cancer cases over-express the Her2…