Press release
Biopharma PEG Develops PEG Linkers for Antibody Drug Conjugates
Antibody-drug conjugate (ADC) is one of the fastest growing fields in tumor therapy, which consists of monoclonal antibody (Antibody), linker (Linker) and active drug (Payload). So far, there are only 15 drugs on the market in the world. However, with the development of some perfect antibody modification techniques, advanced site-specific coupling techniques and powerful small-molecule toxins, ADC drug research has mushroomed and a large number of ADC drugs are in clinical trials.Choosing the most suitable linker is crucial to the ADC development process. The idea of antibody-drug conjugates comes from the "magic bullet" theory proposed by Paul Ehrlich in 1908. The real ADC was not approved for marketing by the US FDA until 2000, that is, Wyeth's gemtuzumab, in which the antibody is a recombinant humanized CD33 monoclonal antibody conjugated with the cytotoxin calicheamicin for the treatment of acute myeloid leukemia. However, through a large number of clinical studies, it was found that it could not improve the survival rate of patients, and had extremely serious side effects, so it was withdrawn from the market in 2010. The first-generation ADC drugs are not strong in targeting tumors, and their localization rate is low. One of the reasons for the failure of Gemtuzumab was that the linker used at that time was chemically unstable, and it was easily hydrolyzed when it did not reach the target, so that the drug was more toxic. As the first generation of ADC, it is also a major breakthrough of ADC. The lessons of its success and failure will affect the development of ADC later, especially for the selection of linker.
A suitable linker helps maintain the stability between the antibody and the drug and helps the antibody selectively deliver the drug to tumor cells and release the drug accurately. PEG is one of the most widely used linkers in targeted therapy. PEG linker has the characteristics of high usage rate, strong targeting, and pH adjustment. On the one hand, drug molecules generally have poor water solubility, adding PEG to the linker structure can increase the water solubility of the entire molecule. On the other hand, an important indicator DAR needs to be considered, that is, the number of drug molecules that can be carried on a unit amount of antibody. PEG can appropriately increase the DAR value, so that the ADC drug can improve the drug efficacy on the basis of low toxicity. In addition, it can also increase the circulating half-life of ADC.
In May 2021, China's CFDA approved goxatuzumab (Trodelvy®, sacituzumab govitecan-hziy) for the treatment of unresectable metastatic disease who have previously received at least 2 systemic therapies or at least 1 of them. Adult patients with locally advanced or metastatic triple-negative breast cancer (mTNBC). The drug was conjugated to the cytotoxic drug SN-38, an active metabolite of irinotecan, via a cleavable maleimide linker with a short PEGylated unit.
Biochempeg is dedicated to provide a chemical synthesis and high-quality PEG linkers. We are committed to promoting the progress of your ADC discovery and development projects. Some featured PEG linkers for ADC from Biopharma PEG as below.
NH2-PEG24-COOH, CAS No.: 196936-04-6
2-((Azido-PEG8-carbamoyl)methoxy)acetic acid, CAS No.: 846549-37-9
Mal-NH-PEG8-COOH, CAS No.: 1334177-86-4
N3-PEG3-SPA, CAS NO.: 1245718-89-1
mPEG36-NH2, CAS NO.: 32130-27-1
OH-PEG8-OH, CAS NO.: 5117-19-1
OH-PEG9-OH, CAS NO.: 3386-18-3
Fmoc-NH-PEG12-COOH, CAS NO.: 1952360-91-6
Biopharma PEG Scientific Inc.
Address: 108 Water Street, Room 4D, Watertown, MA 02472, USA
TEL: 1-857-366-6766
Fax: 617-206-9595
Email: sales@biochempeg.com
Website: https://www.biochempeg.com/
Biopharma PEG Scientific Inc. is a biotechnology-oriented company in Watertown, Massachusetts. We are dedicated to manufacturing and supplying high purity monodispersed and polydispersed polyethylene glycol (PEG) derivatives and PEG raw material, PEGylation services, and custom PEG derivative synthesis to clients worldwide. We continuously expand the capability to provide large-scale manufacture of high purity PEG derivatives with an extensive variety of functional groups, in both non-GMP and GMP grade. These PEG linkers have been widely used in bioconjugation, antibody-drug conjugates (ADCs) therapeutic, click chemistry, 3d bioprinting, drug delivery and diagnostics field, etc.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biopharma PEG Develops PEG Linkers for Antibody Drug Conjugates here
News-ID: 2888027 • Views: …
More Releases from Biopharma PEG Scientific Inc.

Biopharma PEG Supplies Cholesterol (Plant-Derived) Used As Excipients for Lipid …
Cholesterol, a derivative of cyclopentane polyhydrophenanthrene, is the main steroid compound in mammals. Most of the traditional cholesterol comes from animal brainstem and lanolin, which is of animal origin and has the risk of carrying animal viruses. Biopharma PEG innovatively uses plant sterols as starting materials to prepare plant-derived cholesterol (CAS NO.: 57-88-5) through biological fermentation and green synthesis, eliminating the generation and carrying of viruses from the source.
Cholesterol has…
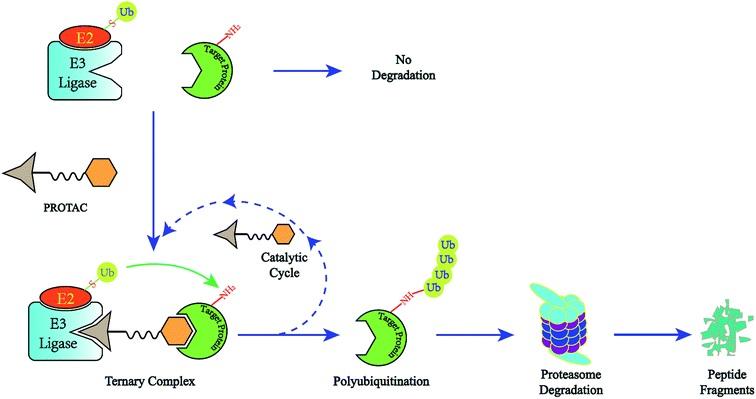
Development Trends And Potential Challenges of PROTACs
PROTAC technology has been in development for more than 20 years. PROTAC proof-of-concept studies date back to 2001, when Crews' team tested the possibility of artificially induced intracellular protein degradation with a peptide that was too large in molecular weight and required cells to penetrate the peptide to improve cell permeability. The discovery of the first small molecule PROTAC and the subsequent small molecule E3 ligand, reported in 2008, greatly…
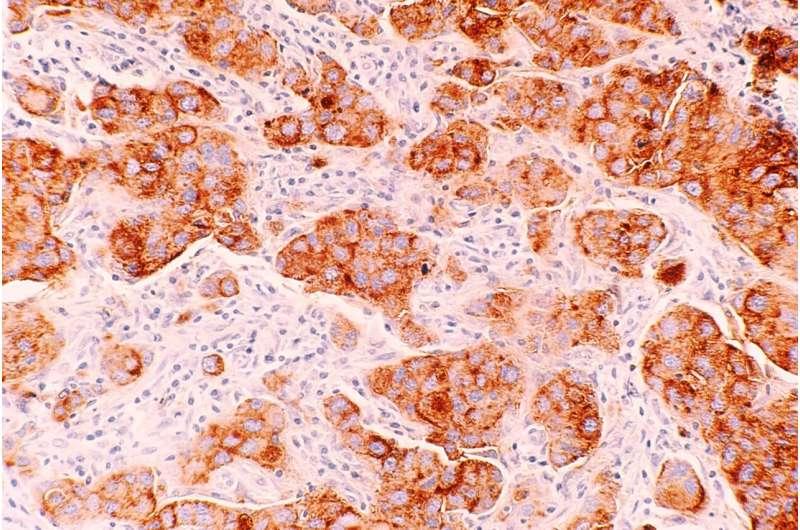
ADC Drugs For Breast Cancer Treatment
Breast cancer is the malignant tumor with the highest morbidity and mortality among women worldwide. At present, the main therapeutic methods include surgery, chemotherapy, radiotherapy, endocrine therapy and targeted therapy, etc. The development and marketing of new drugs have far-reaching significance in improving the survival of breast cancer patients and changing the pattern of breast cancer treatment.
On February 24, 2023, The NMPA approved Enhertu, an injectable drug developed jointly by…
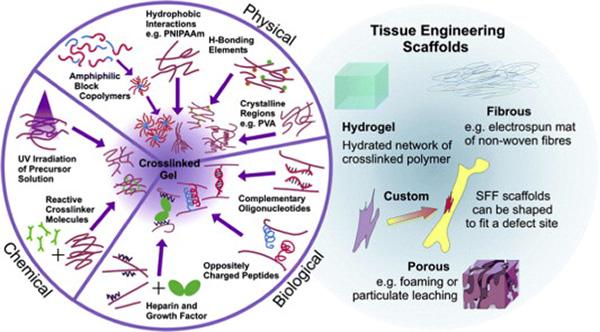
History Development of Hydrogels
Hydrogels are composed of hydrophilic polymers, whose three-dimensional network structure can not only absorb a large amount of water, but also be used to carry drugs. Hydrogels prepared with suitable materials have the characteristics of high biocompatibility, mechanical and viscoelastic control. Since the term was coined in the late 19th century, hydrogels have been widely used in drug delivery, wound dressing, tissue engineering, and hygiene products. This article mainly introduces…
More Releases for ADC
ADC Market: Transforming Cancer Treatment
The global market for antibody-drug conjugates (ADCs) was valued at US$ 11.32 billion in 2023 and is expected to reach US$ 27.37 billion by 2033, growing at a compound annual growth rate (CAGR) of 9.23% from 2024 to 2033. This growth is primarily driven by the increasing prevalence of cancer and the growing demand for safe and effective treatments.
Antibody Drug Conjugates: A Growing Force in Cancer Treatment and Market Expansion
Key…
With Knockout ADC Innovations, Creative Biolabs Took Center Stage at the 2024 Wo …
Creative Biolabs wrapped up a successful show at the 15th Annual World ADC San Diego in the beginning of November, where its third year of participation, highlighting its dedication to the advancement of antibody-drug conjugate (ADC) technology.
New York, USA - November 13, 2024 - Creative Biolabs seized the spotlight at Booth 313 by offering groundbreaking solutions across the entire ADC development spectrum, which demonstrate their unrivaled expertise and dedication to…
Showing Off Their ADC Game: Creative Biolabs Hits the 15th Annual World ADC Summ …
From November 4, Creative Biolabs will be back at the 15th Annual World ADC Summit in San Diego, marking its fourth year at the leading global gathering featuring innovative conjugates.
New York, USA - November 6, 2024 - Creative Biolabs invites all ADC enthusiasts, from new entrants to seasoned experts, to explore its comprehensive suite of ADC solutions and experience the latest in antibody-drug conjugate (ADC) innovation at Booth #313.
Image: https://www.getnews.info/uploads/0c59d26f78bf6dd58aafee5bc68d7d8c.jpg
"2023…
ADC Drugs For Breast Cancer Treatment
Breast cancer is the malignant tumor with the highest morbidity and mortality among women worldwide. At present, the main therapeutic methods include surgery, chemotherapy, radiotherapy, endocrine therapy and targeted therapy, etc. The development and marketing of new drugs have far-reaching significance in improving the survival of breast cancer patients and changing the pattern of breast cancer treatment.
On February 24, 2023, The NMPA approved Enhertu, an injectable drug developed jointly by…
Analog-to-Digital Converters Market by Product (Ramp ADC, Integrating ADC, Succe …
Analog-to-Digital Converters Market by Product (Ramp ADC, Integrating ADC, Successive Approximation ADC, Delta-sigma ADC, and Others (Pipelined/Flash ADC)), and Application (Consumer Electronics and Industrial) - Global Opportunity Analysis and Industry Forecast, 2017-2023
Full Report : https://www.alliedmarketresearch.com/analog-to-digital-converters-market
Increasing disposable income and technological advancements supplement the analog-to-digital converters market. However, complex design of the ADC hampers this market growth. On the other hand, encouragement in digitization of work processes by government in emerging economies,…
Global Data Converters Market Report 2017 (Analog to Digital Converter, Current …
The research report titled Global Data Converters Sales Market Report 2017 market size and forecast and overview on current market trends.
This report studies sales (consumption) of Global Data Converters Market 2017, especially in United States, China, Europe and Japan, focuses on top players in these regions/countries, with sales, price, revenue and market share for each player in these regions, covering
National Semiconductor
Nippon Precision Circuits Inc
Micro Analog systems
Microchip Technology.
TelCom Semiconductor, Inc
Vishay Siliconix
Texas…
