Press release
Huateng Pharma Supplies Some Intermediates of CDK4/6 Inhibitors for Treatment of Advanced Breast Cancer
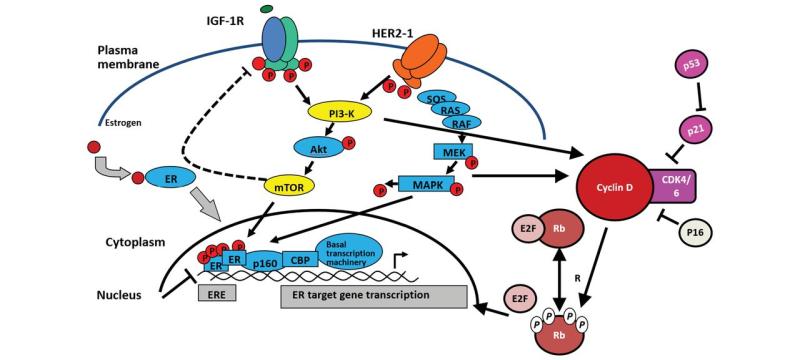
Breast cancer is the most frequent cancer among women, impacting 2.1 million women each year, and also causes the greatest number of cancer-related deaths among women. In 2018, it is estimated that 627,000 women died from breast cancer - that is approximately 15% of all cancer deaths among women. While breast cancer rates are higher among women in more developed regions, rates are increasing in nearly every region globally.The majority of patients with metastatic breast cancer (MBC) have hormone receptor-positive HER2-negative disease. For this subgroup, endocrine therapy is the key therapeutic option. Recently, therapeutic options have been expanded by the introduction of the inhibitors of cyclin-dependent kinases 4/6 (CDK4/6i). Three compounds, palbociclib, ribociclib, and abemaciclib, have already been approved by the FDA for use together with endocrine therapy such as aromatase inhibitors (AIs) or fulvestrant; abemaciclib is also approved as a single agent. Now, let's make a comparison of cdk4/6 inhibitors.
1. Verzenio (Abemaciclib)
According to the approval time from near to far, let's look at Verzenio first. Verzenio is a selective CDK4 and CDK6 inhibitor with IC50 values of 2 nM and 10 nM for CDK4 and CDK6 inhibitory activity, respectively.
The FDA granted Verzenio "breakthrough therapy" status in 2015, and then received Eli Lilly's New Drug Application on May 5, 2017, followed by Verzenio priority review status on July 10, 2017. There are two approved Verzenio therapies. One is combined with fulvestrant to treat female patients with advanced HR-positive and HER2-negative advanced metastatic breast cancer who have deteriorated after receiving endocrine therapy; the other is used as a monotherapy to treat adult patients with advanced metastatic breast cancer who were exacerbated after endocrine therapy and who were HR positive and HER2 negative before chemotherapy. Compared with other drugs of the same type, its characteristic is that it can be used as a monotherapy.
Common side effects that Verzenio may cause include diarrhea, neutropenia, nausea, abdominal pain, infection, fatigue, decreased red blood cell levels (anemia), loss of appetite, vomiting, headache. Serious side effects include diarrhea, neutropenia, liver Examination of blood and coagulation (deep vein thrombosis / pulmonary embolism). In addition, Verzenio may cause harm to developing fetuses and is not suitable for pregnant women.
2. Kisqali (Ribociclib)
Kisqali is the second FDA-approved oral CDK4 / 6 inhibitor with IC50 values of 10 nM and 39 nM for CDK4 and CDK6 inhibitory activity, respectively.
On March 13, 2017, Kisqali, a targeted anticancer drug developed by Novartis, was approved by the FDA. The combination of Kisqali and aromatase inhibitors are the first-line treatment for women with HR-positive and HER2-negative postmenopausal advanced metastatic breast cancer. Kisqali has achieved breakthrough therapy and priority review certification before being approved.
Common side effects seen in patients include neutropenia, nausea, fatigue, diarrhea, leukopenia, hair loss, vomiting, constipation, headache and back pain.
3, Ibrance (Palbociclib)
Ibrance is an FDA-approved CDK4 / 6 inhibitor with IC50 values of 11 nM and 16 nM for CDK4 and CDK6 inhibitory activity, respectively.
Although Pfizer's development of Ibrance has been bumpy. But finally, on February 3, 2015, the FDA accelerated the approval of Ibrance's marketing application, which is used in combination with letrozole to treat postmenopausal metastatic breast cancer patients with estrogen receptor (ER) positive and human epidermal growth factor receptor 2 (HER2) negative. Also prior to approval, Ibrance has achieved breakthrough therapy certification and priority review qualifications. Ibrance's successful launch is a milestone in the development of CDK inhibitors.
Common side effects seen in patients include Nausea, vomiting, loss of appetite, diarrhea, tiredness, weakness, hair loss, mouth sores, or numbness/tingling of arms/legs.
Huateng Pharma, a professional manufacturer of pharmaceutical API and intermediates, provides Palbociclib and Ribociclib key intermediates with high purity.
Palbociclib Intermediates: CAS NO. 571189-16-7, CAS No. 571188-59-5
Ribociclib Intermediates: CAS No.: 733039-20-8, CAS No. 571188-59-5, CAS No. 52092-47-4
Hunan Huateng Pharmaceutical Co. Ltd.
Address: Lugu Business Plaza E1, Yuelu District, Changsha City, Hunan Province, China
Manufacturing Base: Tongguan Kiln, Wangcheng district, Changsha, Hunan, China
Email: sales@huatengusa.com
Website: https://us.huatengsci.com
Zip code: 410205
Telephone: +86 731 89916275
Fax: +86 0731-82251112-818
Huateng Pharma is a leading and professional manufacturer which can provide pharmaceutical intermediates, PEG derivatives, biochemical reagents, APIs, Vitamin D Derivatives and so on. We have a 34,000 square meter manufacturing site with advanced design concept of intelligent manufacturing, which can realize the integration of the production process and accomplish the complete transformation of lab scale - pilot plant - large-scale commercial production
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Huateng Pharma Supplies Some Intermediates of CDK4/6 Inhibitors for Treatment of Advanced Breast Cancer here
News-ID: 3105214 • Views: …
More Releases from Hunan Huateng Pharmaceutical Co. Ltd.

Huateng Pharma Supplies Minoxidil Intermediate 2,4-Diamino-6-chloropyrimidine (C …
Minoxidil was first introduced by Upjohn Company of the United States, and was first used as an oral drug for the treatment of refractory hypertension in the 1970s. In later clinical applications, doctors observed hair regrowth and generalized excessive hair in balding patients, which led to the development of minoxidil preparations.
Minoxidil can increase local blood supply, stimulate the proliferation and differentiation of hair follicle epithelial cells, so as to promote…

Huateng Pharma Supplies Anti-diabetic API Intermediates With Huge Stock
Diabetes is a serious chronic disease characterized by elevated blood sugar concentrations associated with the effects of abnormal beta cell biology on insulin action. Diabetes occurs when the pancreas does not produce enough insulin or the body does not use the insulin it does produce efficiently.
The most common forms of diabetes are type 1 diabetes and type 2 diabetes. Type 1 diabetes is characterized by insufficient insulin production and…
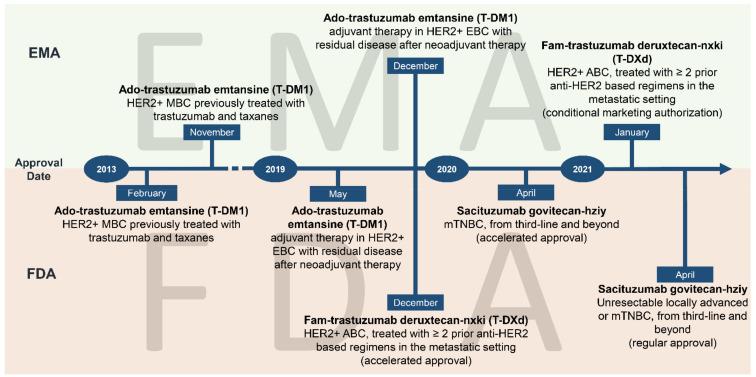
ADC Drugs For HER2 Positive Breast Cancer
According to the latest data, breast cancer has overtaken lung cancer to become the most common cancer among women, and the death rate is the second highest among female tumors, seriously affecting the physical and mental health of women around the world. Patients with abnormal expression of human epidermal growth factor receptor (HER2) account for 15%-20% of all breast cancers, which is highly invasive and has poor prognosis.
Although more drug…

TPD Show Potential For The Treatment of Alzheimer's Disease
Targeted protein degradation (TPD) is a promising strategy in the field of drug discovery. In recent years, targeted protein degradation (TPD) technology has developed rapidly, especially proteolysis targeting chimera (PROTAC), which is the most representative technology of TPD strategy. TPD drugs are one of the hot spots of new drug development in recent years, especially in the field of oncology. For example, a TPD drug developed by Arvinas has achieved…
More Releases for CDK
CDK ELISA Kits Market Outlook and Future Projections for 2030
The cdk elisa kits market represents a dynamic and continually evolving landscape, shaped by changing consumer demands and technological advancements. In this comprehensive report, we provide an in-depth exploration of the market, designed for a wide range of stakeholders including manufacturers, suppliers, distributors, and investors. Our goal is to equip industry participants with essential insights that enable informed decision-making in an ever-changing market environment. This analysis not only examines the…
Dealer Management Services Market Worth Observing Growth | IBM, COX Automotive, …
Advance Market Analytics published a new research publication on "Dealer Management Services Market Insights, to 2030" with 232 pages and enriched with self-explained Tables and charts in presentable format. In the Study you will find new evolving Trends, Drivers, Restraints, Opportunities generated by targeting market associated stakeholders. The growth of the Dealer Management Services market was mainly driven by the increasing R&D spending across the world.
Get inside Scoop of the…
Investigation ongoing for Investors in CDK Global, Inc. (NASDAQ: CDK) over poten …
An investigation on behalf of investors in shares of CDK Global, Inc. (NASDAQ: CDK) was announced over potential breaches of fiduciary duties by certain officers and directors at CDK Global, Inc.
Investors who purchased shares of CDK Global, Inc. (NASDAQ: CDK) have certain options and should contact the Shareholders Foundation at mail@shareholdersfoundation.com or call +1(858) 779 - 1554.
The investigation by a law firm concerns whether certain CDK Global, Inc. directors breached…
Cancer CDK Inhibitors Market 2021 | Detailed Report
According to Market Study Report, Cancer CDK Inhibitors Market provides a comprehensive analysis of the Cancer CDK Inhibitors Market segments, including their dynamics, size, growth, regulatory requirements, competitive landscape, and emerging opportunities of global industry. An exclusive data offered in this report is collected by research and industry experts team.
Get Free Sample PDF (including full TOC, Tables and Figures) of Cancer CDK Inhibitors Market @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=5087783
The report provides a…
Automotive Software Booming Segments; Investors Seeking Growth: CDK Global, Goog …
This intelligence report provides a comprehensive analysis of the Global Automotive Software Market. This includes Investigation of past progress, ongoing market scenarios, and future prospects. Data True to market on the products, strategies and market share of leading companies of this particular market are mentioned. It’s a 360-degree overview of the global market's competitive landscape. The report further predicts the size and valuation of the global market during the forecast…
Cancer CDK Inhibitors Global Industry Report - History, Present and Future 2025
The global market size of Cancer CDK Inhibitors is $XX million in 2017 with XX CAGR from 2013 to 2017, and it is expected to reach $XX million by the end of 2023 with a CAGR of XX% from 2018 to 2023.
There are 3 key segments covered in this report: geography segment, end use/application segment and competitor segment.
For geography segment, regional supply, application-wise and type-wise demand, major players, price is…