Press release
Bayer HealthCare Chooses Werum's PAS-X MES Software
MES Project with focus on Electronic Batch RecordingLueneburg, Germany, June 4, 2008 - Bayer HealthCare launches the implementation of Werum's PAS-X system for its biotech manufacturing processes at its Berkeley, California (USA) location.
In a first step, the Werum Manufacturing Execution System (MES) will be installed in media production. Bayer intends to roll out the MES, which primarily focuses on Electronic Batch Recording (EBR), for running all its manufacturing processes by 2009.
The functional scope of the MES goes far beyond a mere EBR system. The MES will also include applications for Master Batch Record Management (MBR), Weighing & Dispensing (WD), Deviation Management, and Material Tracking. The MES will be integrated with other existing systems, including the SAP system, a distributed control system (DCS), a laboratory information and management system (LIMS), and a document management system (DMS).
Bayer's Berkeley location manufactures the hemophilia medication Kogenate®, which is produced using genetic engineering techniques.
Werum Software & Systems, Tel. USA +1 (973) 644-4000, Tel. Europe +49 (0)4131 8900-0, Press Contact: Volker Mensing, mensing@werum.com
Werum Software & Systems (www.werum.com) is a leading supplier of Manufacturing Execution Systems for the pharmaceutical and biopharmaceutical industry worldwide. Twelve of the world's top 20 pharmaceutical companies use Werum's product suite PAS-X to run their manufacturing business. Outstanding PAS-X features are the functions ensuring compliance and boosting performance. The modular system suite includes components for Production Planning and Scheduling, Master Batch Record Management, Electronic Batch Recording, Weighing and Dispensing, Material Flow Control and Tracking, Equipment Integration, Warehouse Management, Quality Management, and Performance Management based on KPI Analysis. Werum’s solutions and services range from software consulting, creation of functional specifications, and software development to turn-key delivery of integrated and validated Manufacturing Execution Systems. Founded in 1969, the IT company employs more than 290 people in its headquarters in Lueneburg, Northern Germany, the regional offices in West and Southwest Germany, and the subsidiary in Parsippany, New Jersey, USA.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Bayer HealthCare Chooses Werum's PAS-X MES Software here
News-ID: 46265 • Views: …
More Releases from Werum Software & Systems

13th PAS-X User Group Meeting in Germany
13th PAS-X User Group Meeting in Germany
Werum Manufacturing IT Conference with record attendance
Lueneburg, Germany, 26 October 2011 – The 13th international PAS-X User Group Meeting took place from 13 to 14 October 2011 in Lueneburg, Germany, with a record attendance. On the invitation of Werum Software & Systems AG, almost 200 pharmaceuticals experts from approximately 20 countries exchanged their thoughts and ideas on the use of the PAS-X production management…

Indian pharmaceutical major Dr. Reddy's chooses to implement Werum's MES
Dr. Reddy's Laboratories Ltd. deploys PAS-X at Hyderabad site
Lueneburg, Germany, August 20, 2010 – Dr. Reddy's Laboratories Ltd. has decided to introduce PAS-X, the Manufacturing Execution System (MES) by Werum Software & Systems, at its site in Bachupally, Hyderabad.
Dr. Reddy's plans to optimize the efficiency, quality, compliance and performance of its production processes with PAS-X. Werum is the leading supplier of MES systems for the pharmaceutical and biopharmaceutical industries…

MSD Packaging Lines run with PAS-X
Lueneburg, Germany, August 17, 2010 - MSD deployed PAS-X, the Manufacturing Execution System (MES) by Werum Software & Systems, to run its packaging line operations in Heist-op-den-Berg, Belgium in order to increase the compliance level during packaging operations and subsequently shorten lead times to improve delivery performance. Operations include fully automated blister lines and filling lines, semi-automated packaging lines and manual packaging processes. The project for introducing PAS-X was completed…
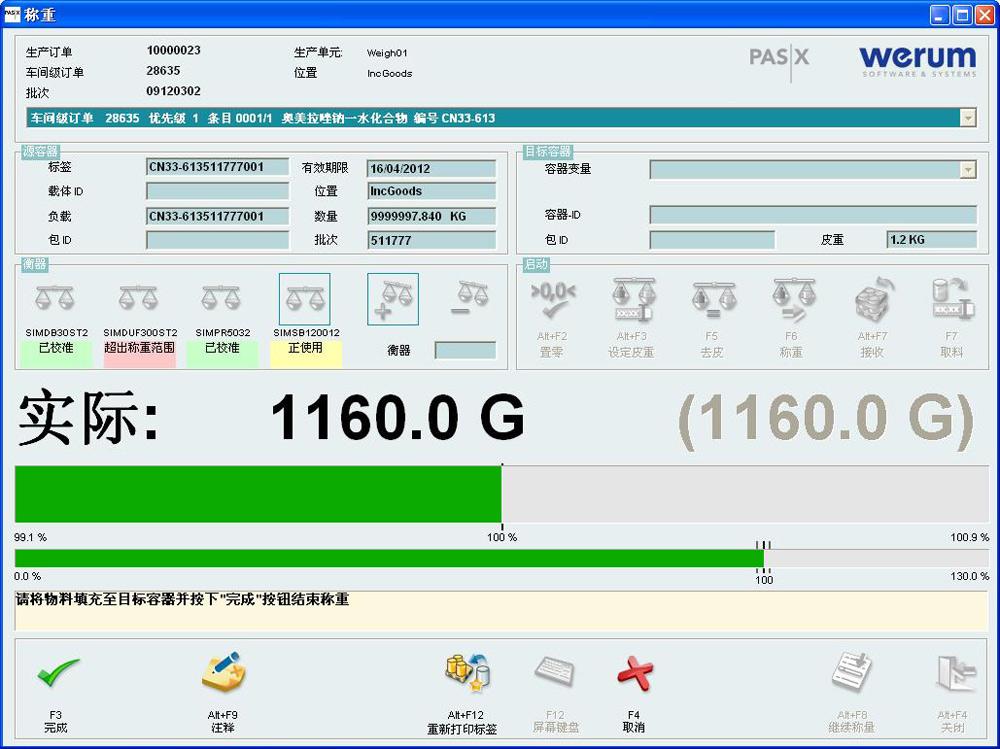
Successful rollout of PAS-X MES Weighing & Dispensing at AstraZeneca in India an …
Manufacturing Execution System projects completed successfully
Lueneburg, Germany, March 25, 2010 - Manufacturing Execution System (MES) software provider Werum Software & Systems has successfully finalized the installation of PAS-X MES in AstraZeneca's Wuxi site in the Jiangsu Province, China. This first-time MES project in the Chinese pharmaceutical industry follows an employment of Werum's PAS-X MES software in AstraZeneca's Indian production site in Bengaluru (formerly Bangalore) in 2009.
Both MES projects are part…
More Releases for MES
PROXIA is ahead with MES!
In our fast-paced world, where progress and technology go hand in hand, engineering software solutions such as MES (Manufacturing Execution Systems) have long been indispensable. But which providers are setting new standards in terms of smooth production processes and efficient management of the entire product life cycle? As part of the "Professional User Rating: Engineering Solutions 2025", the research and analyst firm techconsult, a Heise Group company, examined the MES…
Manufacturing Execution System (MES) Market - The Popularity of Cloud-Based MES …
According to a new market report published by Transparency Market Research The global manufacturing execution system (MES) market is expected to reach a value of US$ 18,067.9 Mn by 2025 on account of the rising demand for improving productivity and executing complex production processes efficiently. The market is projected to expand at a CAGR of 11.2% during the forecast period from 2017 to 2025. The growth is attributed to growing…
Manufacturing Execution System (MES) Market - The Popularity of Cloud-Based MES …
According to a new market report published by Transparency Market Research The global manufacturing execution system (MES) market is expected to reach a value of US$ 18,067.9 Mn by 2025 on account of the rising demand for improving productivity and executing complex production processes efficiently. The market is projected to expand at a CAGR of 11.2% during the forecast period from 2017 to 2025. The growth is attributed to growing…
MES Joins SafeTRANS Network
Berlin (Germany), April 28, 2015: Model Engineering Solutions (MES), specialists in embedded software from Berlin, has become a member of the international competence network SafeTRANS. SafeTRANS is an association of prestigious enterprises and scientific institutions that ensures exchange between research and industry in embedded systems for transportation. The goal of the network is to increase the safety of transportation with the help of cutting-edge technology and optimized processes.
Focus of the…
MES: Expansion in Asia
Berlin, November 24, 2014: Model Engineering Solutions (MES), the Berlin-based specialist for model-based software development, has significantly expanded its business in Asia. In recent years, MES has built up a network of local partners in Japan, Korea, China, and India in order to benefit from the growing market and increasing interest in model-based development and ISO 26262 in Asia.
Asia: Driving force in the automotive industry
The automotive industry continues…
MES & Process Minds Europe 2013
Automation & Control - Improving Operational Excellence in Process Industry
Manufacturing operations by implementing Comprehensive & Compliant Data and Information Platform & Integrated IT systems|
30th September– 1th October 2013, Maritime ProArte Hotel Berlin, Germany
http://pharma-mes2013.we-conect.com/
"Very good and informative conference to share knowledge and to benchmark your company in your industry sector."
Kathrin Wilhelm, Fresenius Kabi D-GmbH
By popular demand, we.CONECT is bringing board-level attendees from manufacturing execution systems management from all over…
