Press release
ADC Drugs For Breast Cancer Treatment
Breast cancer is the malignant tumor with the highest morbidity and mortality among women worldwide. At present, the main therapeutic methods include surgery, chemotherapy, radiotherapy, endocrine therapy and targeted therapy, etc. The development and marketing of new drugs have far-reaching significance in improving the survival of breast cancer patients and changing the pattern of breast cancer treatment.On February 24, 2023, The NMPA approved Enhertu, an injectable drug developed jointly by AstraZeneca and Daiichi Sankyo, for the treatment of adults with unresectable or metastatic HER2-positive breast cancer who have previously received one or more anti-HER2 drugs.
Dertrastuzumab is an antibody-drug conjugate (ADC) drug consisting of a humanized monoclonal antibody targeting HER2 attached to a topoisomerase 1 inhibitor payload via a tetrapeptide cleavable linker. The antibody can be released to partially target and attach to HER2 on cancer cells, and upon entering the cancer cells, release chemotherapeutic drugs to kill the cancer cells as well as nearby cells.
What are ADCs?
ADC drugs combine the high specificity of monoclonal antibody drugs with the high activity of small molecule cytotoxic drugs to improve the targeting of tumor drugs and reduce toxic side effects. ADCs usually consist of a fully humanized monoclonal antibody (mAb), a cytotoxic drug, a suitable linker, and an antigen specifically expressed on tumor cells. In 2000, the FDA approved the first ADC molecule, Gemtuzumab ozogamicin, for the treatment of AML.
Compared with traditional fully or partially humanized antibodies or antibody fragments, ADCs are theoretically more effective due to their ability to release highly active cytotoxins within tumor tissues. Compared with fusion protein, it has higher tolerance and lower side effects. The accurate identification of the target and the non-cancer cell immunity of ADC drugs greatly improve the efficacy and reduce the toxic side effects, which has attracted much attention from the personnel in the field of pharmaceutical research and development.
Currently, 15 ADC drugs have been approved worldwide, including three for breast cancer: T-DM1 and T-DXd, which target human epidermal growth factor receptor 2 (HER2), and sacituzumab govitecan, which targets human trophoblast surface antigen 2 (Trop2).
Trastuzumab Emtansine (T-DM1)
Trastuzumab Emtansine (T-DM1) is the first ADC drug approved for breast cancer. As early as 2013 and 2019, it has been approved by FDA for the adjuvant therapy of HER2-positive advanced breast cancer patients who have received trastuzumab and paclitaxel chemotherapy and HER2-positive early breast cancer patients who have residual HER2-positive early breast cancer patients after receiving taxa and trastuzumab neoadjuvant therapy, respectively.
Trastuzumab Deruxtecan (T-Dxd, DS-8201)
The new generation of T-DXd greatly expands the adaptable population. T-DXd is also an ADC drug targeting HER2, jointly developed by AstraZeneca in the UK and Daiichi Sankyo in Japan. It is trastuzumab (Herceptin, Trastuzumab) and exatecan, through the peptide chain composed of compound drugs. T-DXd has a "bystander effect". While killing HER2-positive cells, it is also killing tumor cells with low HER2 expression.
The results of phase 3 clinical research on breast cancer with "low expression of HER2" show that the treatment effect of T-Dxd is significantly better than that of chemotherapy for breast cancer patients with low expression of HER2! The overall median progression-free survival increased from 5.1 months to 9.9 months, and the median overall survival increased from 16.8 months to 23.4 months! Based on this, T-DXd was approved by the US FDA for marketing.
It was approved in China on February 24, 2023 for the treatment of unresectable or metastatic HER2-positive adult breast cancer patients who have previously received one or more anti-HER2 drugs. It brings more possibilities for patients with low expression of HER2, and will further change the whole pattern of breast cancer treatment in the future.
Sacituzumab Govitecan
Sacituzumab govitecan is composed of an antibody targeting Trop-2 linked to SN-38, the active metabolite of the chemotherapy drug Irinotecan. The full name of TROP2 protein is trophoblast cell surface antigen 2, which is highly expressed in a variety of tumors, such as pancreatic cancer, breast cancer, colon cancer, bladder cancer, oral squamous cell carcinoma and ovarian cancer.
On June 7, 2022, sacituzumab govitecan was officially approved in China for the treatment of patients with unresectable locally advanced or metastatic disease who have received at least two prior systemic therapies (at least one of which is for metastatic disease). Adult patients with negative breast cancer (TNBC). Triple-negative breast cancer treatment ushers in a new era of ADC.
Biopharma PEG is a world leading PEG derivatives supplier that dedicated to supplying high quality ADC linkers & Click Chemistry Reagents and other PEG derivatives to our clients all over the world. ADC linkers with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis.
Biopharma PEG Scientific Inc.
Address: 108 Water Street, Room 4D, Watertown, MA 02472, USA
TEL: 1-857-366-6766
Fax: 617-206-9595
Email: sales@biochempeg.com
Website: https://www.biochempeg.com/
Biopharma PEG Scientific Inc. is a biotechnology-oriented company in Watertown, Massachusetts. We are dedicated to manufacturing and supplying high purity monodispersed and polydispersed polyethylene glycol (PEG) derivatives and PEG raw material, PEGylation services, and custom PEG derivative synthesis to clients worldwide. We continuously expand the capability to provide large-scale manufacture of high purity PEG derivatives with an extensive variety of functional groups, in both non-GMP and GMP grade. These PEG linkers have been widely used in bioconjugation, antibody-drug conjugates (ADCs) therapeutic, click chemistry, 3d bioprinting, drug delivery and diagnostics field, etc.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release ADC Drugs For Breast Cancer Treatment here
News-ID: 2983021 • Views: …
More Releases from Biopharma PEG Scientific Inc.

Biopharma PEG Supplies Cholesterol (Plant-Derived) Used As Excipients for Lipid …
Cholesterol, a derivative of cyclopentane polyhydrophenanthrene, is the main steroid compound in mammals. Most of the traditional cholesterol comes from animal brainstem and lanolin, which is of animal origin and has the risk of carrying animal viruses. Biopharma PEG innovatively uses plant sterols as starting materials to prepare plant-derived cholesterol (CAS NO.: 57-88-5) through biological fermentation and green synthesis, eliminating the generation and carrying of viruses from the source.
Cholesterol has…
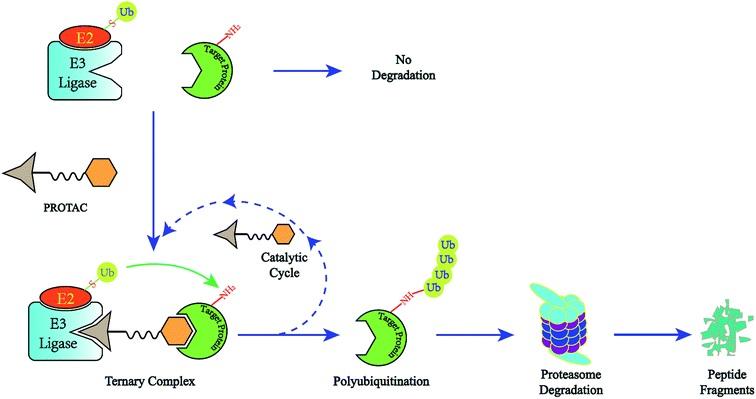
Development Trends And Potential Challenges of PROTACs
PROTAC technology has been in development for more than 20 years. PROTAC proof-of-concept studies date back to 2001, when Crews' team tested the possibility of artificially induced intracellular protein degradation with a peptide that was too large in molecular weight and required cells to penetrate the peptide to improve cell permeability. The discovery of the first small molecule PROTAC and the subsequent small molecule E3 ligand, reported in 2008, greatly…
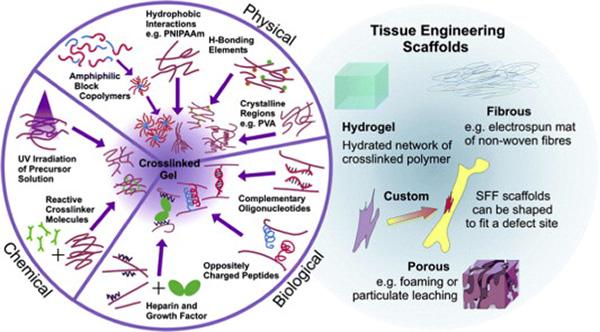
History Development of Hydrogels
Hydrogels are composed of hydrophilic polymers, whose three-dimensional network structure can not only absorb a large amount of water, but also be used to carry drugs. Hydrogels prepared with suitable materials have the characteristics of high biocompatibility, mechanical and viscoelastic control. Since the term was coined in the late 19th century, hydrogels have been widely used in drug delivery, wound dressing, tissue engineering, and hygiene products. This article mainly introduces…

Biopharma PEG Develops PEG Linkers for Antibody Drug Conjugates
Antibody-drug conjugate (ADC) is one of the fastest growing fields in tumor therapy, which consists of monoclonal antibody (Antibody), linker (Linker) and active drug (Payload). So far, there are only 15 drugs on the market in the world. However, with the development of some perfect antibody modification techniques, advanced site-specific coupling techniques and powerful small-molecule toxins, ADC drug research has mushroomed and a large number of ADC drugs are in…
More Releases for HER2
HER2-Positive Breast Cancer (HER2+ BC) Clinical Market to Reach USD 21.46 Billio …
Sub-Headline: The global HER2-Positive Breast Cancer Clinical Market is expected to rise from USD 13.82 billion in 2023 to USD 21.46 billion by 2030, registering a CAGR of 6.5%, driven by rapid uptake of antibody-drug conjugates (ADCs), dual-targeted therapies, and AI-enabled precision oncology diagnostics.
Introduction
The HER2-Positive Breast Cancer (HER2+ BC) Clinical Market is undergoing a major transformation fueled by next-generation targeted therapies, breakthrough ADCs, biosimilar expansion, and genomic testing advancements. HER2+…
Evolving Market Trends In The HER2-Positive Breast Cancer Industry: Innovative T …
Our market reports now include the latest updates on global tariffs, trade impacts, and evolving supply chain dynamics.
What Is the Expected HER2-Positive Breast Cancer Market Size During the Forecast Period?
Over the past few years, there has been a noteworthy expansion in the HER2-positive breast cancer market. The market, which is projected to increase from $10.21 billion in 2024 to $10.96 billion in the following year, predicts a compound annual growth…
HER2 Inhibitors: Advancing Breast Cancer Treatments
"The Business Research Company recently released a comprehensive report on the Global HER2 Inhibitors Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive Sample…
HER2 Positive Breast Cancer Pipeline Drugs 2024
DelveInsight's, "HER2 Positive Breast Cancer Pipeline Insight 2024" report provides comprehensive insights about 60+ companies and 65+ pipeline drugs in HER2 Positive Breast Cancer pipeline landscape. It covers the HER2 Positive Breast Cancer pipeline drug profiles, including clinical and nonclinical stage products. It also covers the HER2 Positive Breast Cancer therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in…
HER2 Inhibitors: Targeted Therapies Transforming Cancer Treatment
HER2 inhibitors market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses the market to account to grow at a CAGR of 9% in the above mentioned forecast period.
Global HER2 Inhibitors Market Scope and Market Size
The HER2 inhibitors market is segmented on the basis of treatment, application, end-users and distribution channel. The growth amongst these segments will help you analyze…
ADC Drugs For HER2 Positive Breast Cancer
According to the latest data, breast cancer has overtaken lung cancer to become the most common cancer among women, and the death rate is the second highest among female tumors, seriously affecting the physical and mental health of women around the world. Patients with abnormal expression of human epidermal growth factor receptor (HER2) account for 15%-20% of all breast cancers, which is highly invasive and has poor prognosis.
Although more drug…