Press release
Huateng Pharma Supplies PEG Products As Ingredients In mRNA COVID-19 Vaccines
Since the COVID-19 epidemic, Pfizer and Moderna have used mRNA technology in a vaccine to help humans fight COVID-19. While mRNA technology is new to the public, the research has been around since the early 1990s.What is an mRNA vaccine?
An mRNA vaccine is a vaccine that uses a molecular copy of messenger RNA (mRNA) to generate an immune response. Such vaccines deliver mRNA molecules into the body's immune cells, where they deliver a specific set of instructions to make protein fragments used by certain viruses. These protein molecules stimulate an adaptive immune response, teaching the body to recognize and destroy the corresponding viral attack. Using this technology, scientists have been experimenting with the potential use of mRNA for deadly diseases such as influenza, Ebola and SARS. Scientists have been experimenting with the use of mRNA therapy to develop personalized cancer treatments, as well as to develop vaccines against infectious diseases such as Zika virus. Scientists are also exploring whether mRNA therapy could be used as a protein replacement therapy for rare diseases such as the blood clotting disorder hemophilia.
mRNA vaccines have developed rapidly in the past two years of the epidemic, and the entire industry chain has now formed a behemoth, including incoming capital, production capacity in the hundreds of millions, and countless related workers.
But it is clear that there is only one product that supports this huge industrial chain - the new crown mRNA vaccine. But as per capita vaccination rates have increased, the market for mRNA vaccines for COVID-19 has declined in various expectations.
If mRNA doesn't find the next support point as soon as possible, it is actually difficult to maintain the "mRNA myth" by relying on the COVID-19 mRNA vaccine alone, so you can see that both Moderna and BioNTech are hurrying to carry out multi-party cooperation or develop new products by themselves.
When we talk about mRNA technology, we have to start with the central principle that DNA transports mRNA, and mRNA is translated into proteins, so as to achieve the cytoplasmic transmission of the genetic material of life.
In mRNA technology, mRNA that can encode a specific protein is delivered directly into human cells so that the cells translate the mRNA to express the target protein, either to activate the immune system's response to the protein (which is the mechanism of mRNA vaccines), or to replenish disease-causing proteins that the body lacks, and so on.
Since mRNA technology can enable cells to produce any information, it has a broad application prospect, including antiviral, tumor, protein deficiency/error diseases, and other types of diseases.
Virus vaccines & Tumor vaccines
In fact, mRNA technology is currently best known for its use in vaccines.
Based on the success of the COVID-19 mRNA vaccine, mRNA projects targeting various viral infections have also been launched, mostly based on the same basic principles.
Design mRNA that encodes viral fragments, and then inject them into the human body, so that cells produce viral proteins, thereby activating the human immune system to fight the virus and establish memory immunity to the disease. Once in the environment, the body is exposed to the virus, the body's immune system is activated again to fight the virus.
mRNA is in the direction of vaccines, in addition to antiviral vaccines, there is also a type of vaccine - tumor vaccines. However, compared with antiviral vaccines, the development of tumor vaccines is still relatively early (the leading echelon is generally in clinical phase 1), and there are more challenges to be solved.
First of all, the target problem of tumor vaccines is more eye-catching. Compared with the single target of virus vaccines, mRNA tumor vaccines usually encode twenty or thirty target proteins. This is also due to the variable target of mutation in tumor cells, while avoiding tumor target loss.
Secondly, mRNA tumor vaccines, as far as we currently understands, are mostly autologous products. This will also lead to a cost and price issue, which we will not discuss much, but can refer to CAR-T cell therapy.
In general, it is better to be optimistic about the next mRNA virus vaccine than to expect the overtaking of mRNA tumor vaccines.
mRNA therapy
The therapeutic use of messenger RNA (mRNA) has fueled great hope to combat a wide range of incurable diseases. Under the assumption of mRNA therapy, many diseases can be treated.
Therapeutic applications include:
(1) Protein replacement to restore the function of a single protein for rare single-gene diseases;
(2) Cell reprogramming, mRNA can regulate cell behavior by expressing transcription or growth factors;
(3) Immunotherapy, in which mRNA-encoded transcripts elicit specific immune responses against target cells, such as therapeutic antibodies, to express virtually any desired protein in host cells and tissues and preserve host cell-intrinsic encoding Post-translational modification of proteins.
For mRNA therapy, low immunogenicity, high stability, and efficient translation are required by design. However, the expression of mRNA vaccines is transient and will degrade over time. Therefore, for chronic diseases, mRNA therapy is a long-term medication regimen.
Secondly, the delivery of mRNA is still dominated by lipid nanoparticles (LNPs). Lipid Nanoparticle (LNP) is currently the dominant delivery system. They are often used in vaccines due to their relatively easy uptake by antigen-presenting cells. The three major mRNA vaccine giants Moderna, CureVac and BioNTech are currently using LNP delivery technology for their COVID-19 vaccines. According to the current research reports, LNP technology is still insufficient and may have potential toxic and side effects. In addition, the current targeting of mRNA-LNPs is dominated by the liver, lung, and heart, and for other organs and cell types, new delivery technologies need to be developed.
In the field of mRNA therapy, CureVac Chief Financial Officer Pierre Kemula said in an interview that the eye may be a good breakthrough, with an independent treatment window and a safer dose.
mRNA gene editing
mRNA gene editing is also an emerging application of mRNA technology. Using mRNA to express Cas9 or other proteins in CRISPR gene editing technology can avoid the risks associated with the integration of nucleases into the host genome.
In this application area, the transient expression of mRNA becomes a unique advantage. The transient nature of mRNA expression limits intracellular nucleases, reducing the risk of off-target cleavage and immune responses to the Cas9 protein.
Conclusion
As a new pharmaceutical technology, mRNA has made breakthroughs in the treatment of infectious diseases and tumors in a short time. As a new technology, mRNA technology still has many areas to be developed, including various application areas and disease treatment. As science continues to advance, mRNA technology is expected to improve more disease treatments in the future.
As a professional PEG derivatives and pharmaceutical intermediates supplier, Huateng Pharma is able to provide high quality PEG products which used as ingredients in mRNA COVID-19 vaccines for your mRNA delivery from mg to kg.
Hunan Huateng Pharmaceutical Co. Ltd.
Address: Lugu Business Plaza E1, Yuelu District, Changsha City, Hunan Province, China
Manufacturing Base: Tongguan Kiln, Wangcheng district, Changsha, Hunan, China
Email: sales@huatengusa.com
Website: https://us.huatengsci.com
Zip code: 410205
Telephone: +86 731 89916275
Fax: +86 0731-82251112-818
Huateng Pharma is a leading and professional manufacturer which can provide pharmaceutical intermediates, PEG derivatives, biochemical reagents, APIs, Vitamin D Derivatives and so on. We have a 34,000 square meter manufacturing site with advanced design concept of intelligent manufacturing, which can realize the integration of the production process and accomplish the complete transformation of lab scale - pilot plant - large-scale commercial production, with an annual capacity of more than 1 billion RMB.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Huateng Pharma Supplies PEG Products As Ingredients In mRNA COVID-19 Vaccines here
News-ID: 2715779 • Views: …
More Releases from Hunan Huateng Pharmaceutical Co. Ltd.

Huateng Pharma Supplies Minoxidil Intermediate 2,4-Diamino-6-chloropyrimidine (C …
Minoxidil was first introduced by Upjohn Company of the United States, and was first used as an oral drug for the treatment of refractory hypertension in the 1970s. In later clinical applications, doctors observed hair regrowth and generalized excessive hair in balding patients, which led to the development of minoxidil preparations.
Minoxidil can increase local blood supply, stimulate the proliferation and differentiation of hair follicle epithelial cells, so as to promote…

Huateng Pharma Supplies Anti-diabetic API Intermediates With Huge Stock
Diabetes is a serious chronic disease characterized by elevated blood sugar concentrations associated with the effects of abnormal beta cell biology on insulin action. Diabetes occurs when the pancreas does not produce enough insulin or the body does not use the insulin it does produce efficiently.
The most common forms of diabetes are type 1 diabetes and type 2 diabetes. Type 1 diabetes is characterized by insufficient insulin production and…
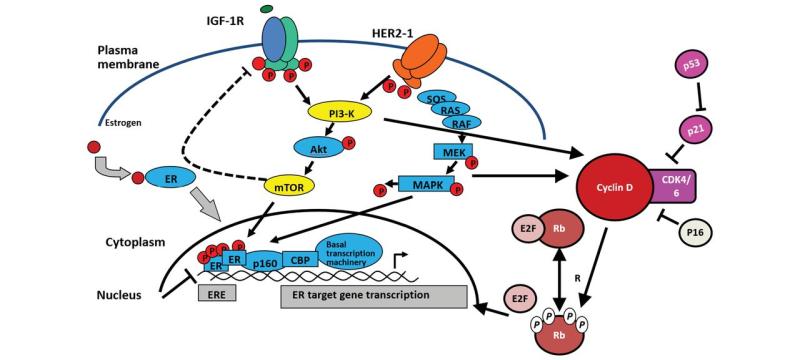
Huateng Pharma Supplies Some Intermediates of CDK4/6 Inhibitors for Treatment of …
Breast cancer is the most frequent cancer among women, impacting 2.1 million women each year, and also causes the greatest number of cancer-related deaths among women. In 2018, it is estimated that 627,000 women died from breast cancer - that is approximately 15% of all cancer deaths among women. While breast cancer rates are higher among women in more developed regions, rates are increasing in nearly every region globally.
The majority…
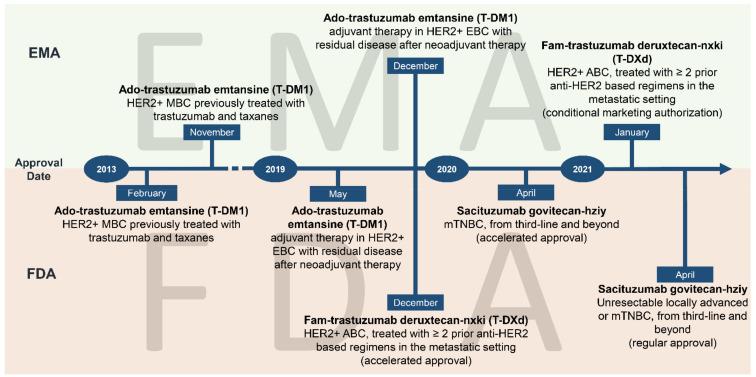
ADC Drugs For HER2 Positive Breast Cancer
According to the latest data, breast cancer has overtaken lung cancer to become the most common cancer among women, and the death rate is the second highest among female tumors, seriously affecting the physical and mental health of women around the world. Patients with abnormal expression of human epidermal growth factor receptor (HER2) account for 15%-20% of all breast cancers, which is highly invasive and has poor prognosis.
Although more drug…
More Releases for RNA
CD Formulation Launches Custom Circular RNA Synthesis Service to Accelerate RNA …
CD Formulation introduces a customizable circRNA synthesis service, delivering high-quality, stable circRNAs for therapeutics, vaccines, and gene research, supported by advanced design and QC processes.
CD Formulation, a leading provider of advanced small nucleic acid synthesis [https://www.formulationbio.com/nucleic-acid/custom-small-nucleic-acid-synthesis.html] solutions, is proud to announce the launch of its fully customizable circular RNA (circRNA) synthesis service. This new service addresses the growing need for stable, non-immunogenic RNA molecules for therapeutic development, vaccine research, and…
Self-Amplifying RNA Synthesis Market Gains Traction as Biotech Firms Embrace Sca …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " Self-Amplifying RNA Synthesis Market- (By Product & Service (Products (Enzymes & Reagents, Premade saRNA, Others), Custom Synthesis Services), By Application (Therapeutics Development (Oncology, Infectious Diseases, Others), Biomedical Research), By End-User (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic,…
RNA Extraction and RNA Purification Market: Growth, Trends & Competitive Landsca …
The global RNA Extraction and RNA Purification Market is expected to grow at 6.3% CAGR from 2025 to 2032.
This Market Report is the result of extensive research and analysis conducted by our team of experienced market researchers through -
• 70% efforts of Primary Research
• 15% efforts of Secondary Research
• 15% efforts from the subscription to Paid database providing industry overview, macro and micro economics factors, and financials of private limited…
RNA Targeting Small Molecules Therapeutics Market: Exponential Growth with Risin …
Estimations Predict a CAGR of 29.8% by 2029 in Global RNA Targeting Small Molecules Therapeutics Market Boosted by Precision Medicine, RNA Biomarker Identification and RNA Genetic Manipulation
What Is The Projected Market Size of The Global RNA Targeting Small Molecules Therapeutics Market And Its Growth Rate?
• The market will grow from $6.1 billion in 2024 to $7.87 billion in 2025 at a compound annual growth rate (CAGR) of 28.9%.
• Expected exponential…
Global DNARNA Extraction Kit Market by Type (Cell-free DNA (cfDNA), Sequence-spe …
"DNARNA Extraction Kit Market" is segmented by Company, Region (country), By Type, Application, stakeholders and other participants. This report provides an analysis of revenue and forecast across Type and Application segments for 2023-2032.
The market for DNARNA Extraction Kits has been thoroughly researched via primary and secondary sources to produce this research study. Along with a competitive analysis of the market, segmented by application, type, and geographical trends, it offers a…
Cancer RNA Expression Market to Reap Excessive Revenues by 2028(By sequencing te …
Worldwide cancer is one of the leading cause of death and effective way of treating it still looks unaccomplished in most parts of the world. The factors which influence the successful treatment of cancer are different depending on the stage of diagnosis, treatment availability and availability of trained healthcare professionals coupled with high economic burden of the disease. The gene expression of cancerous cells varies by cancer type and may…
