Press release
Huateng Pharma Supplies Ensitrelvir Intermeidates Against COVID-19
Ensitrelvir (code name S-217622, brand name Xocova), is a new inhibitor of the SARS-CoV-2 major protease (Mpro), also known as 3C-like protease, has been shown to reduce the viral load and help alleviate the severity of SARS-CoV-2 in infected hamsters. In cells, low nanomolar to sub-micromolar doses of Ensitrelvir suppress viral growth. In hamsters, oral treatment of Ensitrelvir showed excellent pharmacokinetic qualities and hastened recovery from acute SARS-CoV-2 infection.Ensitrelvir also demonstrated antiviral effectiveness against SARS-CoV-2 variants of concern (VOCs), such as the highly pathogenic Delta variant and the newly discovered Omicron variant. Overall, these findings show that Ensitrelvir, which is an antiviral drug that is currently being tested in Phase II/III clinical trials, has impressive antiviral efficiency and effectiveness against SARS-CoV-2 and could be a viable oral treatment option for COVID-19.
The mechanism of action of Ensitrelvir
Like Paxlovid, Ensitrelvir also targets the 3CL protease (3CLpro). These two oral drugs are designed to block the activity of the SARS-CoV-2-3CL protease, thereby blocking the replication of the novel coronavirus. Now, The Ensitrelvir's Phase III clinical trials is completed. It became the first Japanese domestic pill to treat COVID-19 and the third to be regulatorally approved in February 2022 in Japan.
The treatment effects of Ensitrelvir
Results of a clinical trial of Shionogi's oral drug Ensitrelvir in 428 patients over the age of 12 with mild and moderate symptoms showed that the novel coronavirus was undetectable in 80% of patients on day 4 of three days of oral administration. On the 6th day after taking it for 5 days, the new coronavirus in 100% of the people has completely disappeared.
The clinical trial results also showed that after taking Ensiprevir, symptoms such as runny nose, nasal congestion, sore throat, cough, and dyspnea were greatly improved, with minimal side effects. And it has a significant inhibitory effect on the proliferation of the novel coronavirus strain of Omicron. The oral drug Ensitrelvir developed by Shionogi is a pill. Once approved by the Japanese government, it is expected to become an over-the-counter medicine like cold medicine and a powerful weapon against the COVID-19 epidemic.
The applicable groups of Ensitrelvir
Paxlovid is mainly administered in people over the age of 12 with mild symptom and tend to become severe. But for Ensitrelvir, people over the age of 12 who have been diagnosed as positive can take it, regardless of whether there is a tendency for exacerbation, whether they have symptoms or not. It has a wider range of treatments than Paxlovid.
For Ensitrelvir, regardless of whether there is a tendency to become severe or not, can be taken by people over 12 years old who are diagnosed as positive, regardless of whether they have symptoms or not. It has a wider range of treatments than Paxlovid.
Huateng Pharma is a global provider in contract development and manufacturing for intermediates. We can manufacture, scale-up and supply high-quality intermediates and APIs from our China facility. We can provide custom synthesis from R&D to commercial production of Ensitrelvir intermediates as following.
Ensitrelvir (S-217622) Intermediates:
CAS NO.:1360105-53-8 3-Tert-butyl-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione
CAS NO.:1893125-36-4 6-Chloro-2-methyl-2H-indazol-5-amine
CAS NO.:135206-76-7 3-(Chloromethyl)-1-methyl-1H-1,2,4-triazole
CAS NO.:157911-56-3 2,4,5-Trifluorobenzyl bromide
CAS NO.:76-05-1 Trifluoroacetic acid
Lugu Business Plaza E1, Yuelu District, Changsha City, Hunan Province, P.R. China
sally@huatengusa.com
Huateng Pharma is a global provider in contract development and manufacturing for intermediates. With our focus on operational excellence, and the integration of manufacturing operations with EHS, quality assurance, quality control, regulatory, supply chain management and project management, we can manufacture, scale-up and supply high-quality intermediates and APIs from our China facility.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Huateng Pharma Supplies Ensitrelvir Intermeidates Against COVID-19 here
News-ID: 2610028 • Views: …
More Releases from Hunan Huateng Pharmaceutical Co. Ltd.

Huateng Pharma Supplies Minoxidil Intermediate 2,4-Diamino-6-chloropyrimidine (C …
Minoxidil was first introduced by Upjohn Company of the United States, and was first used as an oral drug for the treatment of refractory hypertension in the 1970s. In later clinical applications, doctors observed hair regrowth and generalized excessive hair in balding patients, which led to the development of minoxidil preparations.
Minoxidil can increase local blood supply, stimulate the proliferation and differentiation of hair follicle epithelial cells, so as to promote…

Huateng Pharma Supplies Anti-diabetic API Intermediates With Huge Stock
Diabetes is a serious chronic disease characterized by elevated blood sugar concentrations associated with the effects of abnormal beta cell biology on insulin action. Diabetes occurs when the pancreas does not produce enough insulin or the body does not use the insulin it does produce efficiently.
The most common forms of diabetes are type 1 diabetes and type 2 diabetes. Type 1 diabetes is characterized by insufficient insulin production and…
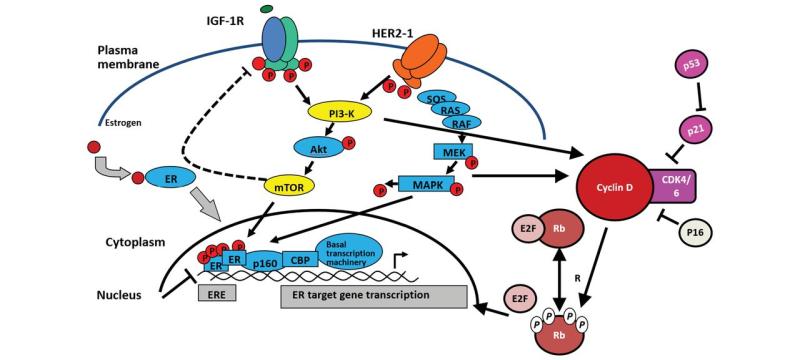
Huateng Pharma Supplies Some Intermediates of CDK4/6 Inhibitors for Treatment of …
Breast cancer is the most frequent cancer among women, impacting 2.1 million women each year, and also causes the greatest number of cancer-related deaths among women. In 2018, it is estimated that 627,000 women died from breast cancer - that is approximately 15% of all cancer deaths among women. While breast cancer rates are higher among women in more developed regions, rates are increasing in nearly every region globally.
The majority…
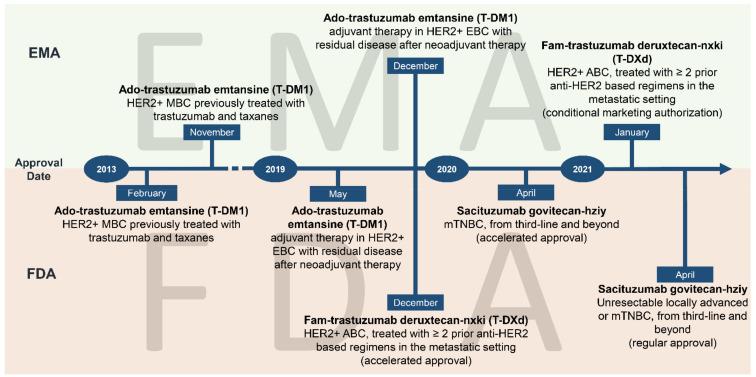
ADC Drugs For HER2 Positive Breast Cancer
According to the latest data, breast cancer has overtaken lung cancer to become the most common cancer among women, and the death rate is the second highest among female tumors, seriously affecting the physical and mental health of women around the world. Patients with abnormal expression of human epidermal growth factor receptor (HER2) account for 15%-20% of all breast cancers, which is highly invasive and has poor prognosis.
Although more drug…
More Releases for Ensitrelvir
Antiviral drugs market is set to reach US$ 127 billion by 2035, North America le …
The global Antiviral drugs market size is estimated to grow from USD 68.94 billion in 2025 to reach USD 73.78 billion in 2026 and USD 127 billion by 2035, representing a higher CAGR of 6.4% during the forecast period 2026 to 2035.
DataM Intelligence unveils its latest report on the "Antiviral drugs Market size 2025," offering an in-depth analysis of market trends, growth drivers, competitive landscape, and regional dynamics. The study…
COVID-19 Mitigation Products Market Overview 2025: Industry Report, Key Players …
DataM Intelligence has published a new research report on "COVID-19 Mitigation Products Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Sample PDF Of This Report…
Global Fumaric Acid Market to Reach USD 1.14 Billion by 2035, Driven by Demand f …
The global fumaric acid market is projected to grow from USD 591.8 million in 2025 to USD 1,142.6 million by 2035, reflecting a steady compound annual growth rate (CAGR) of 6.8% over the forecast period.This growth is fueled by rising demand from the pharmaceutical, food and beverage, and construction industries, where fumaric acid is increasingly valued for its stability, versatility, and eco-friendly properties.
Fumaric Acid Market Outlook
Fumaric acid is a key…
United States Anti-Infective Drugs Market expected to grow at a high CAGR during …
"The Global Anti-Infective Drugs Market is estimated to reach at a high CAGR during the forecast period (2024-2031)." As per DataM intelligence research report
Download your exclusive sample report today: (corporate email gets priority access): https://www.datamintelligence.com/download-sample/anti-infective-drugs-market?sp
United States: Recent Industry Developments
✅ In November 2025, Recce Pharmaceuticals announced patent approval for its synthetic anti-infectives (RECCE® 327/R327 and RECCE® 529/R529) covering novel treatment pathways for bacterial, viral, and multi-drug resistant infections. The patents strengthen…
Fumaric Acid Prices, Trends & Forecasts | Provided by Procurement Resource
Fumaric acid prices followed diverse trends in different regions during the first half of 2023. In Asia, prices initially declined due to lower feedstock prices but later found support from reduced supply caused by manufacturing unit shutdowns. However, demand slowdown in the second quarter led to stockpiling and decreased production. Europe saw an increase in prices in the first quarter due to supply-demand balance and higher costs but experienced a…
Fumaric Acid Prices, Trends & Forecasts | Provided by Procurement Resource
Fumaric acid prices followed diverse trends in different regions during the first half of 2023. In Asia, prices initially declined due to lower feedstock prices but later found support from reduced supply caused by manufacturing unit shutdowns. However, demand slowdown in the second quarter led to stockpiling and decreased production. Europe saw an increase in prices in the first quarter due to supply-demand balance and higher costs but experienced a…
