Press release
Dolutegravir is a first-line treatment for newly diagnosed HIV patients
A new study by UBC researchers is set to change international treatment recommendations for people who are newly diagnosed with HIV--an update that could affect nearly two million people per year worldwide. The study found that dolutegravir is the optimal medication for first-line treatment for people newly diagnosed with HIV, a choice that has not been clear over the past several years.Dolutegravir (DTG), sold under the brand name Tivicay, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS. It may also be used, as part of post exposure prophylaxis, to prevent HIV infection following potential exposure. The risk of adverse reaction meant that, although dolutegravir was found to be favorable compared to other options, it was only recommended as an alternative, with an antiretroviral called efavirenz recommended as the primary treatment.
The study team completed a network meta-analysis of research stemming from 68 available antiretroviral therapy (ART) clinical trials. They found dolutegravir was superior to efavirenz in most outcomes, including viral suppression, tolerability, and safety. According to Kanters, the increased odds of viral suppression with dolutegravir could have a significant impact on achieving international goals for HIV treatment.
"We found about a five per cent increase in the probability of viral suppression, which means that more people who start treatment will be able to successfully control their HIV," he said.
Another key attribute of dolutegravir is that it is effective in people who are resistant to NNRTI-class antiretrovirals, like efavirenz, a problem that is becoming increasingly common.
"Dolutegravir appears to be here to stay as the preferred treatment for people newly diagnosed with HIV," he said. "However, it is important to recognize the good that efavirenz has done over the past two decades, as it helped lead the ART scale-up around the world."
Despite the many benefits of dolutegravir, dolutegravir use was associated with increased weight gain, a side effect that could increase the risk of aging-associated comorbidities, like heart attack or stroke.
"In many places, well-treated HIV has become a chronic condition and we are now seeing people living long lives with HIV," said Kanters. "The research community will continue to monitor the effects dolutegravir may have on the healthy aging process."
While this study is specifically focused on the optimal treatment for people newly diagnosed with HIV, an upcoming publication will review the evidence in support of switching to dolutegravir for people whose first treatment choice has been unsuccessful in controlling their infection.
This recommendation could mean improved treatment for the many people living with HIV around the world who are unable to achieve viral suppression despite being on treatment.
Huateng Pharma is known worldwide for a variety of pharmaceutical intermediates used in research and development. We can provide anti-virus drug intermediates Dolutegravir intermediates for your research. We can make scale-up production with capacities varying from gram to kilograms and multi tons.
Address:Lugu Business Plaza E1, Yuelu District, Changsha City, Hunan Province, China
Email: sales@huatengusa.com
Website:https://us.huatengsci.com
Zip code: 410205
Telephone: +86 731 89916275
Human Huateng Pharmaceutical Co., Ltd. is a leading and professional manufacturer which can provide pharmaceutical intermediates, PEG derivatives, biochemical reagents, APIs, Vitamin D Derivatives and so on. We are committed to providing cost-effective pharmaceutical products and services to customers around the world with the belief of “Strive for the Human Health".
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Dolutegravir is a first-line treatment for newly diagnosed HIV patients here
News-ID: 2470722 • Views: …
More Releases from Hunan Huateng Pharmaceutical Co. Ltd.

Huateng Pharma Supplies Minoxidil Intermediate 2,4-Diamino-6-chloropyrimidine (C …
Minoxidil was first introduced by Upjohn Company of the United States, and was first used as an oral drug for the treatment of refractory hypertension in the 1970s. In later clinical applications, doctors observed hair regrowth and generalized excessive hair in balding patients, which led to the development of minoxidil preparations.
Minoxidil can increase local blood supply, stimulate the proliferation and differentiation of hair follicle epithelial cells, so as to promote…

Huateng Pharma Supplies Anti-diabetic API Intermediates With Huge Stock
Diabetes is a serious chronic disease characterized by elevated blood sugar concentrations associated with the effects of abnormal beta cell biology on insulin action. Diabetes occurs when the pancreas does not produce enough insulin or the body does not use the insulin it does produce efficiently.
The most common forms of diabetes are type 1 diabetes and type 2 diabetes. Type 1 diabetes is characterized by insufficient insulin production and…
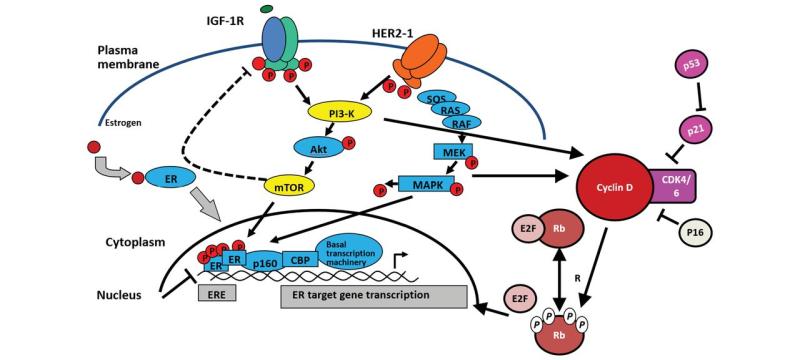
Huateng Pharma Supplies Some Intermediates of CDK4/6 Inhibitors for Treatment of …
Breast cancer is the most frequent cancer among women, impacting 2.1 million women each year, and also causes the greatest number of cancer-related deaths among women. In 2018, it is estimated that 627,000 women died from breast cancer - that is approximately 15% of all cancer deaths among women. While breast cancer rates are higher among women in more developed regions, rates are increasing in nearly every region globally.
The majority…
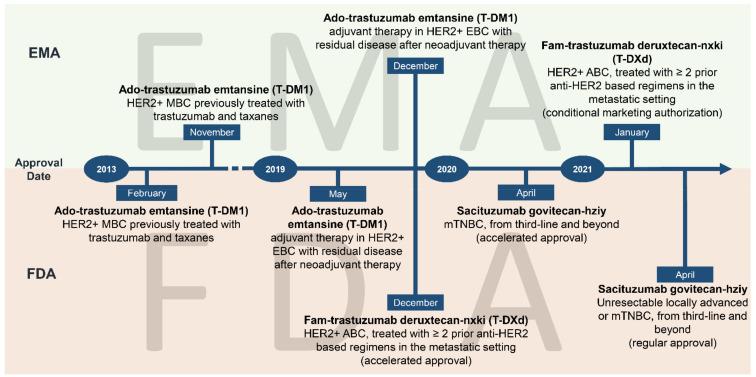
ADC Drugs For HER2 Positive Breast Cancer
According to the latest data, breast cancer has overtaken lung cancer to become the most common cancer among women, and the death rate is the second highest among female tumors, seriously affecting the physical and mental health of women around the world. Patients with abnormal expression of human epidermal growth factor receptor (HER2) account for 15%-20% of all breast cancers, which is highly invasive and has poor prognosis.
Although more drug…
More Releases for HIV
HIV Drugs Market - Defeating HIV Together: Advancing Treatment Options for a Bri …
Newark, New Castle, USA: The "HIV Drugs Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
HIV Drugs Market: https://www.growthplusreports.com/report/hiv-drugs-market/7792
This latest report researches the industry structure, sales, revenue,…
HIV-Associated Lipodystrophy Treatment Market - Increasing prevalence of HIV is …
HIV-associated lipodystrophy also known as lipodystrophy is a syndrome that occurs in HIV-infected patients. It is characterized by loss of subcutaneous fat from face, buttocks, arms and legs. Although the exact cause of HIV-associated lipodystrophy is not fully elucidated, some research evidence reported that it occurs in HIV-infected patients who are under antiretroviral medications. According to an article published in National Center for Biotechnology Information (NCBI) in 2014, prevalence of…
Global HIV Drugs Market | Global HIV Drugs Industry | Global HIV Drugs Market Re …
Human immunodeficiency Virus (HIV) could be a chronic and severe sickness which might be transferred from one person to a different through blood-to-blood and sexual contact. it's a deadly disease that attacks immune cells called CD-4 cells, creating body vulnerable to infections and alternative diseases. Over the years, the rising prevalence of HIV sickness worldwide has completely influenced the demand for HIV medicine. HIV medicine facilitate in preventing the multiplication…
HIV Therapeutics Market– South Africa's Aspen launches three-in-one HIV drug
Recent Developments
Aspen Pharma care, a South Africa’s drug maker has launched a triple combination of tablet for the treatment of HIV in the country where the HIV virus is the most prevalent. The company's new Emdolten drug is a once a day tablet which is in the form of dolutegravir, an antiretroviral medication that balances the drug’s resistance. The company has launched Aspen Stavudine which was its first generic ARV…
HIV Vaccine Market HIV Vaccine Clinical Pipeline Report 2022
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
Introduction to Human Immunodeficiency Virus (HIV) Vaccines
1.1 Overview
1.2 Antiquity of HIV Vaccine
Need for the Development of HIV Vaccine
Primer of HIV inside the Body
3.1 Inclusion of HIV Virus into the System
3.2 Interaction of HIV with Host
3.3 Eradication of HIV Virus
HIV Vaccine Development Process
4.1 Introduction
…
Global HIV Vaccine Market & HIV Vaccine Clinical Trial Outlook 2022
Worldwide, around the 35 Million of the people are currently infected with the HIV and about 30 Million of the people died because of the AIDS infection. There is no human example of clearing an HIV infection naturally. HIV virus makes copies of it very quickly, many types of HIV exist and new types of virus are continue to rise. Many scientists are still trying to understand the specific ways…