Press release
Cytocentrics receives GLP certification
The Ministry for Health and Social Affairs of the state of Mecklenburg-Vorpommern awarded Cytocentrics AG the GLP certificate (Good Laboratory Practice) category 9 on 3rd of April. The Rostock-based biotechnology company is the first company in the new federal states of Germany with the authorization to carry out safety pharmacological tests on human ion channels in accordance with nationally and internationally recognized GLP guidelines.Cytocentrics’ customers are pharmaceutical companies that wish to avoid undesired side effects of pharmaceutical ingredients with human ion channels. This type of testing is required by the EMEA, the European regulatory authority for pharmaceutical drugs as well as its US counterpart, the FDA. These authorities’ regulations stipulate that all non-clinical trials must be carried out under the GLP guidelines. “This serves to clarify side effects of substances before they are administered to people”, explained Dr. Thomas Knott, board member and founder of Cytocentrics AG.
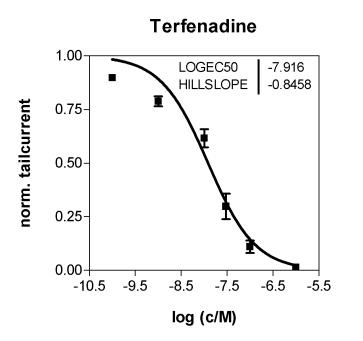
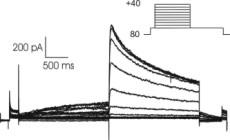
Cytocentrics AG is now authorized to perform one part of this pre-clinical testing specific for the effects of pharmaceutical substances on human ion channels as a service provider. “One unique aspect of our certification is that our automated patch clamp technology, called CytoPatch™, has been approved in addition to the conventional patch clamp method. This technology has been in-house at Cytocentrics,” commented Dr. Knott.
GLP is a quality assurance system which governs the planning, carrying out and monitoring of non-clinical safety tests. The strict GLP standard was originally introduced to enable mutual acceptance of the results of studies performed by different laboratories in different countries. The carrying out of studies, as well as the documentation and archiving of the resulting data are strictly regulated. The quality system also has strict requirements concerning employee training and technical equipment. Authorities carry out regular audits of companies to verify the implementation of the GLP quality standard.
Cytocentrics AG
Dr. Christa Nutzhorn
Joachim-Jungius-Str. 9
18059 Rostock
Germany
Tel: +49(0)381 4059-640
E-mail: info@cytocentrics.com
Cytocentrics AG is a biotechnology company applying its ion channel expertise to support the pharmaceutical industry in the drug discovery process. The products are designed to simplify and optimize laboratory productivity. Cytocentrics want to make the drug discovery process more efficient and cost effective while focusing on quality and reliability.
The contribution is to provide cutting-edge solutions to assist drug screening of ion channel targets, especially in early drug profiling and safety pharmacology.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Cytocentrics receives GLP certification here
News-ID: 41985 • Views: …
More Releases from Cytocentrics AG
CCS Cell Culture Service GmbH and Cytocentrics AG agree cooperation in the field …
The Biotechnology companies CCS Cell Culture Service GmbH and Cytocentrics AG today announced the signing of a research and license agreement. The aim of the cooperation is to develop electrophysiologically optimized ion channel cell lines which allow a reliable testing of substances and drugs.The pharmaceutical industry depends on suitable in-vitro test methods for preclinical drug screening and safety pharmacology testing. Over recent years the automated patch clamp technology has established…
Constant cell quality in compliance with high standards in safety pharmacology
The CytoPAQ™ Instant Cells Kit replaces time consuming and costly cell cultivation. This innovative system, developed by Cytocentrics AG, consists of certified HEK 293 cells stably transfected with hERG (human ether-a-go-go related gene)channel, intracellular and extracellular solution, and Cell Reservoir (optional accessory). CytoPAQ™ is a validated system in regards to PAQ (Performance, Accuracy and Quality) and lays the foundation for reliable and reproducible data generation.The Instant Cells are ready to…
More Releases for GLP
GLP Diet Review: Tailored Nutrition and Support for GLP-1 Users
Image: https://www.globalnewslines.com/uploads/2026/01/1768225059.jpg
GLP Diet Review - A Personalized GLP-1 Diet
The GLP Diet [https://glp.diet/?utm_source=abnewswire&utm_medium=organic&utm_campaign=bio] is a personalized wellness program designed to help users maximize the benefits of their GLP-1 weight loss medications. It offers tailored meal plans, balanced recipes, guided workouts, lifestyle challenges, and practical tools for tracking progress. Built around each user's unique needs, it turns complex nutrition advice into clear daily actions that support weight loss, better health, and long-term…
Alpharetta Medical Clinic Earns Top Recognition for Excellence in Peptide, GLP-1 …
Dr. Weight Loss of Atlanta in Alpharetta is proud to announce its recognition as the top-rated provider of medical weight loss and peptide therapy services in the Alpharetta area. This distinction reflects the clinic's sustained commitment to clinical excellence, patient-centered care, and successful treatment outcomes in the field of metabolic health.
Alpharetta, GA - December 16, 2025 - Dr. Weight Loss of Atlanta [https://drweightlossofatlanta.com/peptide-therapy-alpharetta] in Alpharetta is proud to announce its…
Medical Clinic Recognized as Top Peptide, GLP-1, & GLP-3 Therapies in Sandy Spri …
Dr. Weight Loss of Atlanta announced today that its Sandy Springs location has earned recognition as a leading medical weight loss and peptide therapy clinic in Sandy Springs, reflecting exceptional patient satisfaction, consistently strong clinical outcomes, and a reputation for delivering personalized, expert-guided care.
Sandy Springs, GA - December 16, 2025 - Dr. Weight Loss of Atlanta [https://drweightlossofatlanta.com/peptides-sandy-springs] announced today that its Sandy Springs location has earned recognition as a leading…
My Start GLP 1 Reviews: The GLP 1 That is Changing the Way America loss Weight
In the modern era of health innovation, few breakthroughs have generated as much excitement as My Start GLP 1. In 2025, it has rapidly become one of the most talked-about doctor-supervised weight loss programs in the United States. With thousands of verified patient success stories, My Start GLP 1 has positioned itself as more than a trend-it is a science-backed revolution changing how we understand metabolism, hunger, and sustainable fat…
GLP-1 Analogues Latest Market Analysis Report 2025
Global Info Research announces the release of the report "Global GLP-1 Analogues Market 2025 by Manufacturers, Regions, Type and Application, Forecast to 2031". This report provides a detailed overview of the GLP-1 Analogues market scenario, including a thorough analysis of the GLP-1 Analogues market size, sales quantity, average price, revenue, gross margin and market share.The GLP-1 Analogues report provides an in-depth analysis of the competitive landscape, manufacturer's profiles, regional and…
GLP-1 Market Taming Type 2 Diabetes and Obesity: The Therapeutic Promise of the …
GLP-1 Market Worth $95.4 Mn by 2031 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global GLP-1 Market Size, Share & Trends Analysis Report By Type of Molecule (Biologics and Small Molecules), Active Compound Used (Dulaglutide, Liraglutide, Orforglipron, Retatrutide, Semaglutide, Survodutide, Tirzepatide and Other Active Compounds),
Type of GLP-1 Agonist Drugs (Long-acting GLP-1 Agonist and Short-acting GLP-1 Agonist), Type…