Press release
CCS Cell Culture Service GmbH and Cytocentrics AG agree cooperation in the field of cell line development
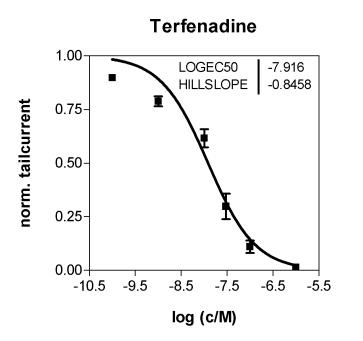
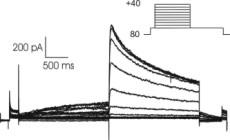
Inhibition of the hERG channel by Terfenadine. The CytoPatch™ instrument for automated patch clamp and hERG-HEK 293 Instant Cells were used to collect data from the common developed cell line.
About CCS Cell Culture Service GmbH (www.cellcultureservice.com)
Since 2000, the CCS Cell Culture Service GmbH based in Hamburg has established itself as provider of cell-based reagents for the screening of potential drugs and the production of recombinant cell lines and has built up a large customer base. Within a few years the company has established itself as partner to many companies in Europe and the USA.
About Cytocentrics AG (www.cytocentrics.com)
Since 2001, the Rostock-based Cytocentrics AG specialises in the development and marketing of innovative solutions for ion channel screening. The company´s portfolio includes an instrument for automated patch clamp, ready-to-use instant cells and a service sector. Customers are mainly pharmaceutical companies in Europe and the USA.
CCS Cell Culture Service GmbH
Dr. Oliver Klotzsche
Falkenried 8
820251 Hamburg
Germany
Phone: + 49 (0)40 471 965 60
Email:Klotzsche@CellCultureService.com
URL: www.cellcultureservice.com
Cytocentrics AG
Dr. Christa Nutzhorn
Joachim-Jungius-Str. 9
18059 Rostock
Germany
Phone: +49(0)381 4059-640
Email: info@cytocentrics.com
URL: www.cytocentrics.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release CCS Cell Culture Service GmbH and Cytocentrics AG agree cooperation in the field of cell line development here
News-ID: 55595 • Views: …
More Releases from Cytocentrics AG
Cytocentrics receives GLP certification
The Ministry for Health and Social Affairs of the state of Mecklenburg-Vorpommern awarded Cytocentrics AG the GLP certificate (Good Laboratory Practice) category 9 on 3rd of April. The Rostock-based biotechnology company is the first company in the new federal states of Germany with the authorization to carry out safety pharmacological tests on human ion channels in accordance with nationally and internationally recognized GLP guidelines.
Cytocentrics’ customers are pharmaceutical companies that wish…
Constant cell quality in compliance with high standards in safety pharmacology
The CytoPAQ™ Instant Cells Kit replaces time consuming and costly cell cultivation. This innovative system, developed by Cytocentrics AG, consists of certified HEK 293 cells stably transfected with hERG (human ether-a-go-go related gene)channel, intracellular and extracellular solution, and Cell Reservoir (optional accessory). CytoPAQ™ is a validated system in regards to PAQ (Performance, Accuracy and Quality) and lays the foundation for reliable and reproducible data generation.The Instant Cells are ready to…
More Releases for Cell
Cell Sorting Market Accelerates as Cell Therapy, Immuno-Oncology & Single-Cell R …
The rising focus on precision medicine, immunotherapy, and advanced cell-based research is driving the global cell sorting market into a high-growth phase. With expanding applications in stem cell therapy, CAR-T manufacturing, cancer immunology, and single-cell genomics, demand for accurate, high-purity cell isolation systems is stronger than ever. This release highlights key market trends, segmentation insights, technological innovations, and the factors shaping the future of cell sorting.
Download Full PDF Sample Copy…
Cell Isolation Cell Separation Market Size Analysis by Application, Type, and Re …
According to Market Research Intellect, the global Cell Isolation Cell Separation market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The market for cell isolation and separation is expanding rapidly as a result of sophisticated biotechnological…
Cell Free Protein Synthesis Market Beyond the Cell: Revolutionizing Protein Prod …
Cell-Free Protein Synthesis Market to reach over USD 457.13 Mn by the year 2031 - Exclusive Report by InsightAce Analytic
"Cell-Free Protein Synthesis Market" in terms of revenue was estimated to be worth $265.94 Mn in 2023 and is poised to reach $457.13 Mn by 2031, growing at a CAGR of 7.20% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for free Sample Pages: https://www.insightaceanalytic.com/request-sample/1445
Current…
Cell Expansion Market - Expand the Boundaries of Cell Therapy: Redefine Cell Exp …
Newark, New Castle, USA: The "Cell Expansion Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cell Expansion Market: https://www.growthplusreports.com/report/cell-expansion-market/7939
This latest report researches the industry structure, sales, revenue,…
Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect …
Researchmoz added Most up-to-date research on "Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect Cell and Others" to its huge collection of research reports.
This report researches the worldwide GMP Cell Banking market size (value, capacity, production and consumption) in key regions like North America, Europe, Asia Pacific (China, Japan) and other regions.
This study categorizes the global GMP Cell Banking breakdown data by manufacturers, region, type…
Cell Culture Market Size, Cell Culture Market Share, Cell Culture Market Trends …
According to a new research published by Polaris Market Research the global cell culture market is anticipated to reach more than USD 49 billion by 2026. Cell culture is a rapidly emerging as an implement for analyzing and treating various disease such as Alzheimer’s and cancer.
Request for Sample of This Research Report @ https://bit.ly/2D7pZ5u
Top Key Players: -
Becton,
Dickinson and Company
Biospherix
EMD Millipore
Eppendorf AG
Merck KGaA
Sartorius AG
VWR International
Cell culture is a rapidly emerging…