Press release
In safe hands with the GLP checklist
The "Good Laboratory Practice" regulatory mandate (GLP for short) was introduced in 1978 after the FDA (U.S. Food and Drug Administration) had already identified significant deficiencies in toxicological studies in the years preceding. The German Chemicals Act was introduced in an effort to ensure that newly approved drugs for human consumption really are safe and minimize any and all risks.The values of transparency, traceability, assignment of responsibilities, and safekeeping are particularly important in this context. The GLP also checks personal, organizational, and spatial requirements before approving a new drug.
To achieve GLP compliance, specific requirements must be met by the laboratory units (e.g., climate chambers) used in the development of the drugs concerned. Maintenance documents and repair documents must be available to consult when a new drug is being tested. To ensure adherence to all rules and regulations on the unit side during the approval process, BINDER has developed a very helpful checklist for its customers. The checklist guides users through the most important points that are relevant to the compliance of their workflows. Questions include, for example: Is a continuous CO2 supply ensured? Are there back-up chambers available for emergencies? Has a cleaning plan or a maintenance plan been drawn up? All of these questions will help users who are working with a simulation chamber and wish to ensure compliance with the quality guidelines of the GLP regulation.
BINDER temperature, climate, and environmental simulation chambers are the centerpieces of modern laboratories. Designed to meet the highest demands in scientific and industrial fields, they enable the accurate simulation of biological, chemical, and physical environmental conditions. With cutting-edge technology and pioneering innovations, BINDER develops products that provide real added value for the customer. We apply our expertise and feel for the requirements of dynamic, day-to-day work in research facilities to support the widest range of projects and processes in an optimum way. After all, our aim is nothing less than making a contribution to the health, quality of life, and safety of people.
Anne Lenze
PR und Event Specialist
BINDER Central Services GmbH & Co. KG
Im Mittleren Ösch 5
D-78532 Tuttlingen
Phone: +49 7462 / 2005 - 632
Fax: +49 7462 / 2005 - 93 632
E-Mail: Anne.Lenze@binder-world.com
www.binder-world.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release In safe hands with the GLP checklist here
News-ID: 1153087 • Views: …
More Releases from BINDER GmbH
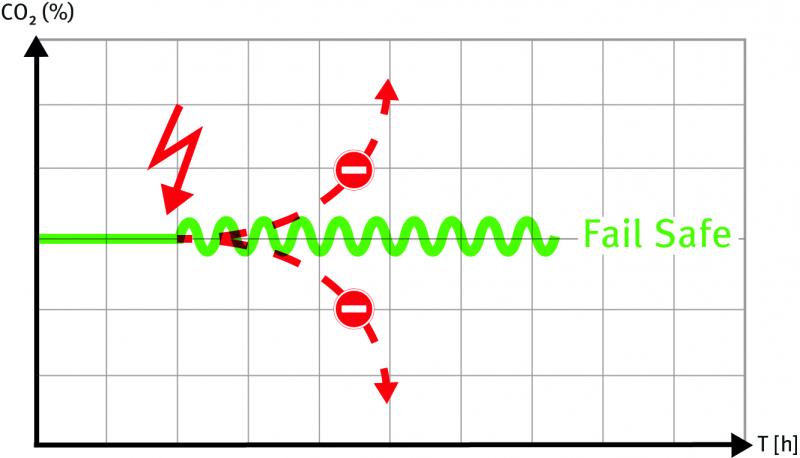
A new innovation from BINDER: The fail-safe function
BINDER incubators are the best on the market. Why is this? There is a simple answer in the first instance: Because they are constructed in as straightforward a way as possible, they are incredibly reliable.
The risk of the cultures inside a BINDER incubator becoming contaminated is reduced to a minimum.
But BINDER customers have high standards and want to know more about the details. This is exactly where the…
More Releases for GLP
GLP-1 SuperDefender: The Revolutionary Oral Protection Appliance Against "Ozempi …
Los Angeles, California - Feb 18, 2026 - As medications like Ozempic Registered , Wegovy Registered , and Mounjaro Registered continue to reshape the future of weight management and diabetes treatment, millions of patients are unknowingly facing a growing dental crisis. Reports of acid reflux, dry mouth, and teeth grinding are rising among GLP-1 medication users, leading to irreversible enamel erosion and tooth loss; Collectively known as "Ozempic Teeth".
In response…
American Made GLP-1 Names CoreAge Rx As Best GLP-1 Provider for 2026
Image: https://www.abnewswire.com/upload/2026/02/8efbd0ec583b0fd3f145d91eb24f2e05.jpg
American Made GLP-1, a US- based healthcare resource dedicated to reviewing and comparing GLP-1 treatment providers, has released its latest rankings of telehealth platforms offering physician-supervised GLP-1 programs for weight management, naming CoreAge Rx as the #1 GLP-1 Provider and awarding the platform an outstanding 4.9 out of 5 rating. The independent review evaluated more than 10 U.S.-based telehealth providers and recognized CoreAge Rx with the designation "Editor's Choice,"…
GLP Diet Review: Tailored Nutrition and Support for GLP-1 Users
Image: https://www.globalnewslines.com/uploads/2026/01/1768225059.jpg
GLP Diet Review - A Personalized GLP-1 Diet
The GLP Diet [https://glp.diet/?utm_source=abnewswire&utm_medium=organic&utm_campaign=bio] is a personalized wellness program designed to help users maximize the benefits of their GLP-1 weight loss medications. It offers tailored meal plans, balanced recipes, guided workouts, lifestyle challenges, and practical tools for tracking progress. Built around each user's unique needs, it turns complex nutrition advice into clear daily actions that support weight loss, better health, and long-term…
Alpharetta Medical Clinic Earns Top Recognition for Excellence in Peptide, GLP-1 …
Dr. Weight Loss of Atlanta in Alpharetta is proud to announce its recognition as the top-rated provider of medical weight loss and peptide therapy services in the Alpharetta area. This distinction reflects the clinic's sustained commitment to clinical excellence, patient-centered care, and successful treatment outcomes in the field of metabolic health.
Alpharetta, GA - December 16, 2025 - Dr. Weight Loss of Atlanta [https://drweightlossofatlanta.com/peptide-therapy-alpharetta] in Alpharetta is proud to announce its…
Medical Clinic Recognized as Top Peptide, GLP-1, & GLP-3 Therapies in Sandy Spri …
Dr. Weight Loss of Atlanta announced today that its Sandy Springs location has earned recognition as a leading medical weight loss and peptide therapy clinic in Sandy Springs, reflecting exceptional patient satisfaction, consistently strong clinical outcomes, and a reputation for delivering personalized, expert-guided care.
Sandy Springs, GA - December 16, 2025 - Dr. Weight Loss of Atlanta [https://drweightlossofatlanta.com/peptides-sandy-springs] announced today that its Sandy Springs location has earned recognition as a leading…
My Start GLP 1 Reviews: The GLP 1 That is Changing the Way America loss Weight
In the modern era of health innovation, few breakthroughs have generated as much excitement as My Start GLP 1. In 2025, it has rapidly become one of the most talked-about doctor-supervised weight loss programs in the United States. With thousands of verified patient success stories, My Start GLP 1 has positioned itself as more than a trend-it is a science-backed revolution changing how we understand metabolism, hunger, and sustainable fat…