Press release
N-Nitroso Phenylephrine: Ensuring Pharmaceutical Safety with Advanced Solutions By Aquigen Bio Sciences to Combat Nitrosamine Contamination Risks, Enhance Product Integrity, and Meet Stringent Regulatory Standards for Safer Healthcare Outcomes
N-Nitroso Phenylephrine, a nitrosamine impurity, has emerged as a significant safety concern in the pharmaceutical industry. As regulatory authorities worldwide heighten their scrutiny of nitrosamine contaminants, pharmaceutical companies must prioritize understanding, detection, and mitigation of these hazardous compounds to ensure product safety and patient health.Learn more about N-Nitroso Phenylephrine: https://aquigenbio.com/product/n-nitroso-phenylephrine/
Understanding N-Nitroso Phenylephrine:
Nitrosamines, including N-Nitroso Phenylephrine, are chemical compounds classified as probable human carcinogens by the International Agency for Research on Cancer (IARC). They can form during the manufacturing process, often due to reactions involving secondary or tertiary amines and nitrosating agents under specific conditions. Phenylephrine, a widely used nasal decongestant and vasoconstrictor, is particularly susceptible to nitrosamine contamination when exposed to these reactive environments.
Hazards of N-Nitroso Phenylephrine:
The presence of N-Nitroso Phenylephrine in pharmaceutical products poses severe health risks. Research indicates that prolonged exposure to nitrosamines, even in trace amounts, can lead to genetic mutations and increase cancer risk. Regulatory guidelines have established Acceptable Daily Intake (ADI) limits for nitrosamines, but the potential accumulation of these impurities across different medications intensifies the concern.
Patients reliant on medications containing phenylephrine could unknowingly be exposed to harmful nitrosamine levels if adequate controls are not implemented. Moreover, nitrosamine contamination not only endangers patient safety but also threatens pharmaceutical companies with regulatory penalties, product recalls, and reputational damage.
Regulatory Expectations and Challenges:
In recent years, global regulatory bodies such as the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO) have issued stringent guidelines to control nitrosamine impurities.
These regulations require pharmaceutical manufacturers to:
1. Identify Risk Factors: Assess raw materials, production processes, and potential sources of nitrosamine formation.
2. Implement Robust Testing: Employ advanced analytical methods to detect and quantify nitrosamines like N-Nitroso Phenylephrine.
3. Develop Mitigation Strategies: Modify processes, reformulate products, or establish stringent quality controls to eliminate or minimize contamination.
Despite these directives, the detection and management of N-Nitroso Phenylephrine remain a formidable challenge due to its chemical complexity and trace-level presence. This underscores the urgent need for innovative solutions and collaboration across the pharmaceutical sector.
Get in Touch with Us - https://aquigenbio.com/contact-us/
The Role of Analytical Science in Combating N-Nitroso Phenylephrine:
Addressing the risks of N-Nitroso Phenylephrine requires cutting-edge analytical techniques and expertise. Methods such as Gas Chromatography-Mass Spectrometry (GC-MS), Liquid Chromatography-Mass Spectrometry (LC-MS), and High-Performance Liquid Chromatography (HPLC) have proven instrumental in detecting nitrosamines with high precision. These technologies enable manufacturers to meet stringent regulatory requirements and ensure the safety of their pharmaceutical products.
Moreover, the adoption of predictive modeling tools can help manufacturers anticipate nitrosamine formation during the early stages of product development. By integrating these methodologies into their workflows, companies can proactively safeguard against contamination risks.
Why Pharmaceutical Companies Must Pay Attention
The pharmaceutical industry's reputation hinges on trust and reliability. Failure to address nitrosamine contamination, including N-Nitroso Phenylephrine, can lead to significant consequences:
1. Health Risks to Patients: Undetected impurities can result in adverse health outcomes, eroding public trust in medications.
2. Regulatory Penalties: Non-compliance with nitrosamine guidelines can lead to warnings, bans, or even market withdrawals.
3. Financial Repercussions: Product recalls, litigation, and loss of consumer confidence can have severe economic impacts.
4. Ethical Responsibility: Pharmaceutical companies have a moral obligation to provide safe and effective medications to their consumers.
As the scrutiny on nitrosamines intensifies, pharmaceutical manufacturers must invest in research, adopt advanced analytical technologies, and foster a culture of compliance and vigilance to mitigate risks associated with N-Nitroso Phenylephrine.
Aquigen Bio Sciences - Your Trusted Partner in Nitrosamine Control:
In the face of mounting regulatory demands and complex safety challenges, pharmaceutical companies need a reliable partner to navigate the intricate landscape of nitrosamine impurities. Aquigen Bio Sciences stands as a leading resource in addressing N-Nitroso Phenylephrine contamination, offering comprehensive solutions tailored to meet industry needs.
With expertise in impurity standard synthesis, advanced analytical testing, and regulatory compliance support, Aquigen Bio Sciences empowers pharmaceutical manufacturers to ensure the safety and integrity of their products. By collaborating with Aquigen Bio Sciences, companies can stay ahead of evolving regulations, protect patient health, and uphold their commitment to quality.
N-Nitroso Phenylephrine is a pressing issue that demands immediate attention and decisive action. Together with trusted partners like Aquigen Bio Sciences, the pharmaceutical industry can overcome these challenges, paving the way for a safer and more reliable future in healthcare.
For more information on N-Nitroso Phenylephrine impurity detection and control solutions, contact Aquigen Bio Sciences today.
Similar Trending Products:
1) N-Nitroso Rivaroxaban Open-Ring Acid Impurity: https://aquigenbio.com/product/n-nitroso-rivaroxaban-open-ring-acid-impurity/
2) N-Nitroso Ropinirole: https://aquigenbio.com/product/n-nitroso-ropinirole/
3) N-Nitroso -N-Demethyl Roxithromycin: https://aquigenbio.com/product/n-nitroso-n-demethyl-roxithromycin/
Contact Us:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen Bio Sciences:
Aquigen Bio Sciences is a renowned contract research organization based in Pune, India, specializing in impurity standards and comprehensive solutions for the pharmaceutical industry. With a focus on advancing pharmaceutical safety and compliance, Aquigen provides expert guidance on identifying, quantifying, and managing impurities in various drug formulations. Their commitment to quality and regulatory excellence positions them as a trusted partner for manufacturers striving to meet stringent safety standards and improve patient outcomes.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release N-Nitroso Phenylephrine: Ensuring Pharmaceutical Safety with Advanced Solutions By Aquigen Bio Sciences to Combat Nitrosamine Contamination Risks, Enhance Product Integrity, and Meet Stringent Regulatory Standards for Safer Healthcare Outcomes here
News-ID: 3762250 • Views: …
More Releases from Aquigen Biosciences
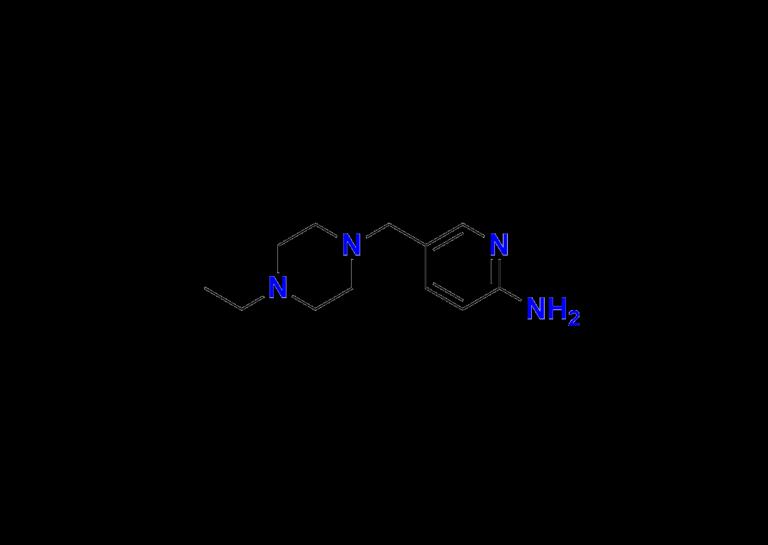
Precision Standards for Oncology Research: Exploring Abemaciclib Impurity 1 and …
In the ever-evolving field of targeted cancer therapy, Abemaciclib has emerged as a pivotal agent in the treatment of hormone receptor-positive (HR+), HER2-negative advanced or metastatic breast cancer. As researchers and pharmaceutical developers continue to innovate in oncology, the importance of impurity profiling and the availability of reliable Abemaciclib impurity standards has never been greater.
At the forefront of pharmaceutical impurity standards, Aquigen Bio is proud to support global manufacturers, CROs,…
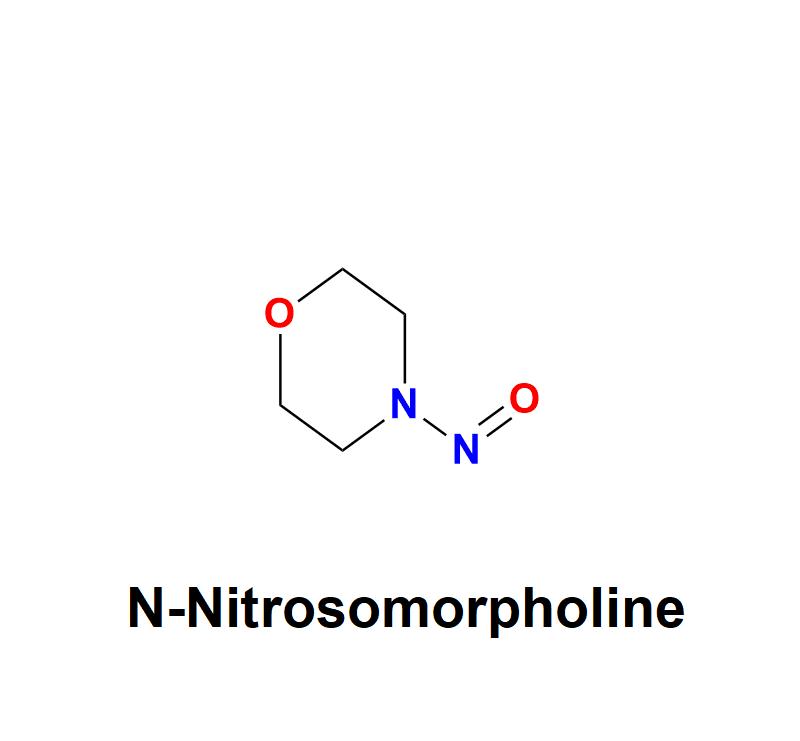
N-Nitrosomorpholine: Addressing Pharmaceutical Safety Challenges with Aquigen Bi …
N-Nitrosomorpholine, a compound belonging to the nitrosamine family, has garnered significant attention in the pharmaceutical and healthcare industries due to its potential carcinogenic risks. This chemical impurity, often found as a byproduct in manufacturing processes, poses serious challenges to drug safety and human health, necessitating stringent monitoring and control measures from pharmaceutical companies.
Learn more about N-Nitrosomorpholine: https://aquigenbio.com/product/n-nitrosomorpholine/
Understanding N-Nitrosomorpholine:
N-Nitrosomorpholine is a nitrosamine impurity characterized by its chemical structure, which includes…
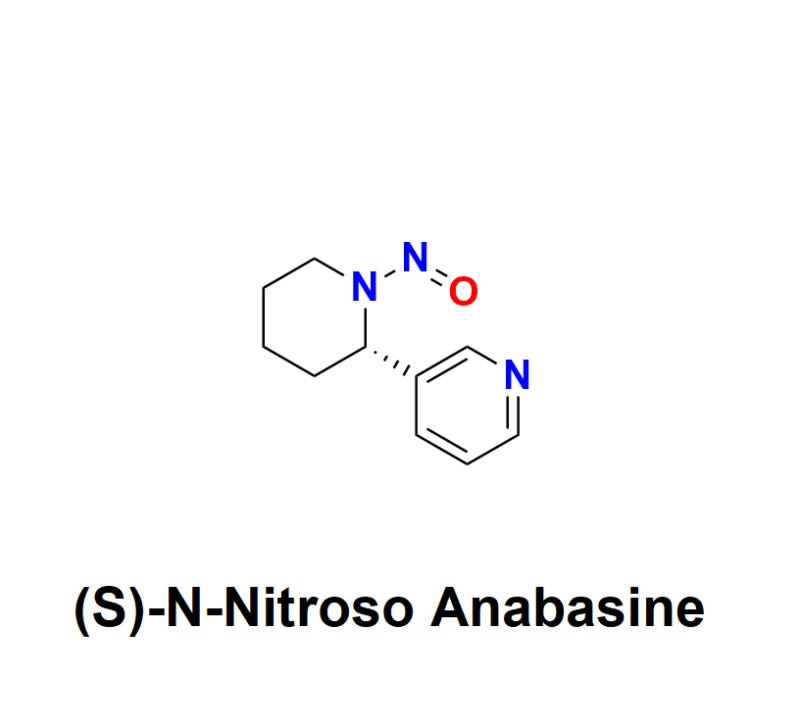
(S)-N-Nitroso Anabasine: Understanding the Risks, Regulatory Challenges, and How …
(S)-N-Nitroso Anabasine, a nitrosamine impurity, has raised significant safety concerns within the pharmaceutical industry. Recognized as a probable human carcinogen, this impurity has become a focal point for global regulatory agencies and manufacturers alike, urging a renewed emphasis on detection, prevention, and management.
Learn more about (S)-N-Nitroso Anabasine: https://aquigenbio.com/product/s-n-nitroso-anabasine/
What Is (S)-N-Nitroso Anabasine?
(S)-N-Nitroso Anabasine belongs to the family of nitrosamines, compounds formed through a chemical reaction known as nitrosation. This…
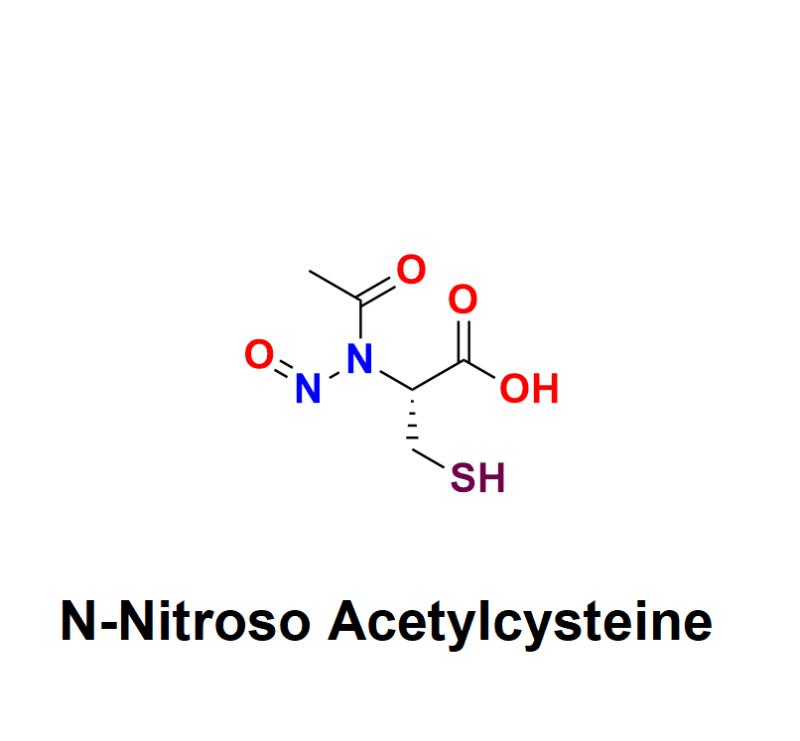
N-Nitroso Acetylcysteine: A Critical Concern in Pharmaceuticals - Exploring Haza …
N-Nitroso Acetylcysteine has emerged as a critical topic of concern in the pharmaceutical industry. As a member of the nitrosamine family, it is a potential impurity that poses significant health risks, including carcinogenicity, even in trace amounts. With increasing regulatory scrutiny on nitrosamine impurities, pharmaceutical manufacturers must address the presence of compounds like N-Nitroso Acetylcysteine to protect public health and ensure compliance with global standards.
Learn more about N-Nitroso Acetylcysteine: https://aquigenbio.com/product/n-nitroso-acetylcysteine/…
More Releases for Phenylephrine
Future of the Phenylephrine Drugs Market: Trends, Innovations, and Key Forecasts …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
Phenylephrine Drugs Market Size Growth Forecast: What to Expect by 2025?
The market for phenylephrine drugs has experienced substantial growth in the past few years. From a market worth of $25.44 billion in 2024, it is projected to rise to $28.03 billion in 2025, exhibiting a compound annual growth…
Phenylephrine Hydrochloride Market Booming Worldwide With Leading Key Players -
The objective of the market research report on the phenylephrine hydrochloride Market is to deliver a comprehensive analysis of the market, featuring insightful observations, statistics, historical data, and industry-validated insights. Additionally, it aims to provide forecasts based on robust assumptions and methodologies. This study enhances the understanding of global phenylephrine hydrochloride market dynamics and structure by segmenting and analyzing various market sectors while estimating the overall market size.
Furthermore, the…
Phenylephrine Hydrochloride Market 2023 Global Industry Size, Segments, Share an …
The global "Phenylephrine Hydrochloride Market" research report (2022-2030) offers exact data regarding business development, future growth strategies, and trend forecasts. Along with sales trends, market size, price structure, market share, and market advancements, it covers the state of the market today and its prospects for the future. The market report for phenylephrine hydrochloride provides a comprehensive review of the global industry's revenue, demand situation, competitive environment, and regional market segments.…
Phenylephrine Market Report- Size, Growth, Analysis Of Key-players Types And App …
The Phenylephrine Market research reports provide industry dynamics and in-depth Phenylephrine Market segmentation with historical, current, and projected industry size along with industry trends. Furthermore, the Phenylephrine Market research report includes an in-depth market analysis utilizing Porter's five force analysis and offers the impact of covid-19 on the market during the forecasted period.
Download FREE PDF of the Report @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=5480692
Phenylephrine market is segmented by players, region (country), by Type and…
Phenylephrine API Market Size, Share, Report & Segmentation 2027 | Valuates Repo …
In 2020, the global Phenylephrine API market size was US$ XX million and it is expected to reach US$ XX million by the end of 2027, with a CAGR of XX% during 2021-2027. In Japan, the Phenylephrine API market size is expected to grow from US$ XX million in 2020 to US$ XX million by 2027, at a CAGR of XX% during the forecast period. Phenylephrine is a decongestant that…
Phenylephrine API Market Company Insights & SWOT Analysis by 2027 | Synergene, J …
This Phenylephrine API market report segmented the market by form, application, product, geography, and other factors. This market report looks at a few main players and drivers that have an effect on market opportunities, challenges, risks, and development. It also conducts a competitive analysis of the industry, which helps main market participants in terms of large profits. The market growth is heavily influenced by the significant factors listed in…
