Press release
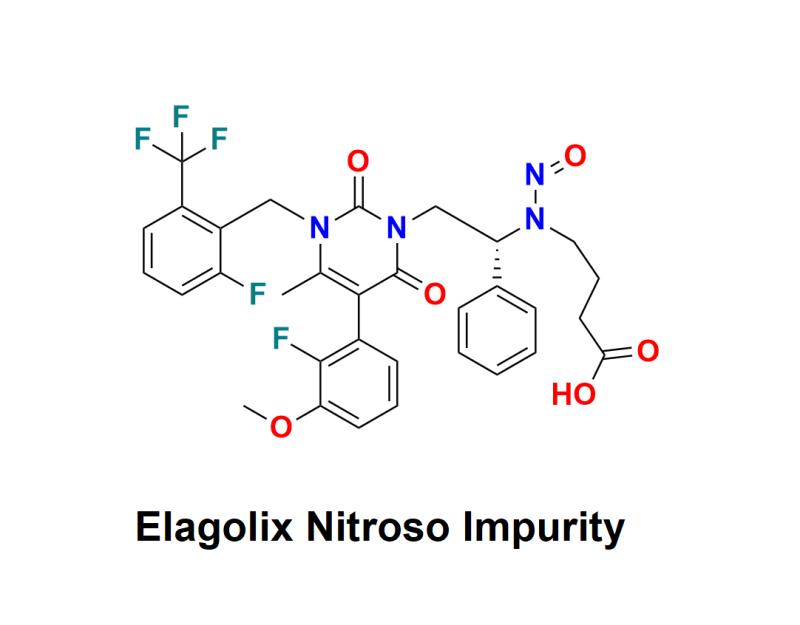
Elagolix Nitroso Impurity: An Essential Quality Standard by Aquigen BioSciences for Enhancing Pharmaceutical Safety, Supporting Analytical Method Development, and Ensuring Compliance with Regulatory Guidelines for Laboratories and Manufacturers
Elagolix Nitroso Impurity signifies an important advancement in the pharmaceutical sector, aimed at laboratories and pharmaceutical companies focused on ensuring the quality and safety of their products. As the industry faces increased scrutiny regarding impurities, the availability of such high-quality standards is crucial for maintaining compliance with regulatory requirements.Elagolix Nitroso Impurity serves as a vital reference material for analytical laboratories involved in the evaluation of Elagolix, a medication primarily used for managing endometriosis and uterine fibroids. This impurity standard aids in the detection and quantification of N-Nitroso Elagolix impurity within pharmaceutical formulations, ensuring that final products meet safety standards and regulatory guidelines.
Learn more about Elagolix Nitroso Impurity: https://aquigenbio.com/product/elagolix-nitroso-impurity/
N-Nitroso compounds, including N-Nitroso Elagolix, are recognized for their potential carcinogenic effects, leading global regulatory bodies such as the FDA and EMA to impose strict limits on their presence in pharmaceutical products. The use of this standard enables researchers and quality control teams to conduct thorough testing, contributing to the assurance of safety and efficacy in pharmaceutical products.
Function and Use:
Elagolix Nitroso Impurity is primarily utilized during the development and quality assurance stages of drug production. Its applications include:
1. Analytical Method Development: Laboratories can use this standard to establish validated analytical methods for detecting and quantifying impurities in Elagolix formulations.
2. Quality Control: This standard plays a critical role in routine quality control processes, facilitating consistent monitoring of impurity levels to ensure compliance with regulatory standards.
3. Stability Testing: The standard can be employed in stability studies to assess the behavior of Elagolix formulations over time, confirming that impurities do not exceed acceptable levels as the product ages.
Get in Touch with Us - https://aquigenbio.com/contact-us/
Promoting Safety in Pharmaceuticals:
Aquigen Bio Sciences is committed to advancing pharmaceutical safety through the provision of innovative solutions. Elagolix Nitroso Impurity exemplifies its dedication to providing high-quality reference materials that meet the stringent demands of the pharmaceutical industry.
With extensive expertise in impurity standards, Aquigen Bio Sciences positions itself as a key resource for pharmaceutical companies and research institutions. They offer a wide range of impurity standards along with comprehensive support services, ensuring that clients have the necessary tools to navigate the complexities of regulatory compliance.
Conclusion:
The launch of the Elagolix Nitroso Impurity represents a significant development in the pharmaceutical industry's commitment to safety and efficacy. By providing this essential resource, Aquigen Bio Sciences enables laboratories and manufacturers to enhance product quality while complying with regulatory requirements.
Similar Trending Products:
1) (S)-3-(Boc-amino)piperidine: https://aquigenbio.com/product/s-3-boc-aminopiperidine-2/
2) N-Nitrosomorpholine: https://aquigenbio.com/product/n-nitrosomorpholine/
3) 6-Amino-1-methyl-5-nitrosouracil: https://aquigenbio.com/product/6-amino-1-methyl-5-nitrosouracil-2/
Contact Us:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen Bio Sciences:
Aquigen Bio Sciences is a leading contract research organization specializing in impurity standards and analytical services for the pharmaceutical industry. With a focus on quality and innovation, the company provides a comprehensive range of reference materials, including the Elagolix Nitroso Impurity. Their commitment to advancing pharmaceutical safety and efficacy makes them a trusted partner for laboratories and manufacturers striving to meet stringent regulatory demands.
For more information about the Elagolix Nitroso Impurity and other products and services, please visit Aquigen Bio Sciences or contact them directly.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Elagolix Nitroso Impurity: An Essential Quality Standard by Aquigen BioSciences for Enhancing Pharmaceutical Safety, Supporting Analytical Method Development, and Ensuring Compliance with Regulatory Guidelines for Laboratories and Manufacturers here
News-ID: 3711456 • Views: …
More Releases from Aquigen Biosciences
Precision Standards for Oncology Research: Exploring Abemaciclib Impurity 1 and …
In the ever-evolving field of targeted cancer therapy, Abemaciclib has emerged as a pivotal agent in the treatment of hormone receptor-positive (HR+), HER2-negative advanced or metastatic breast cancer. As researchers and pharmaceutical developers continue to innovate in oncology, the importance of impurity profiling and the availability of reliable Abemaciclib impurity standards has never been greater.
At the forefront of pharmaceutical impurity standards, Aquigen Bio is proud to support global manufacturers, CROs,…
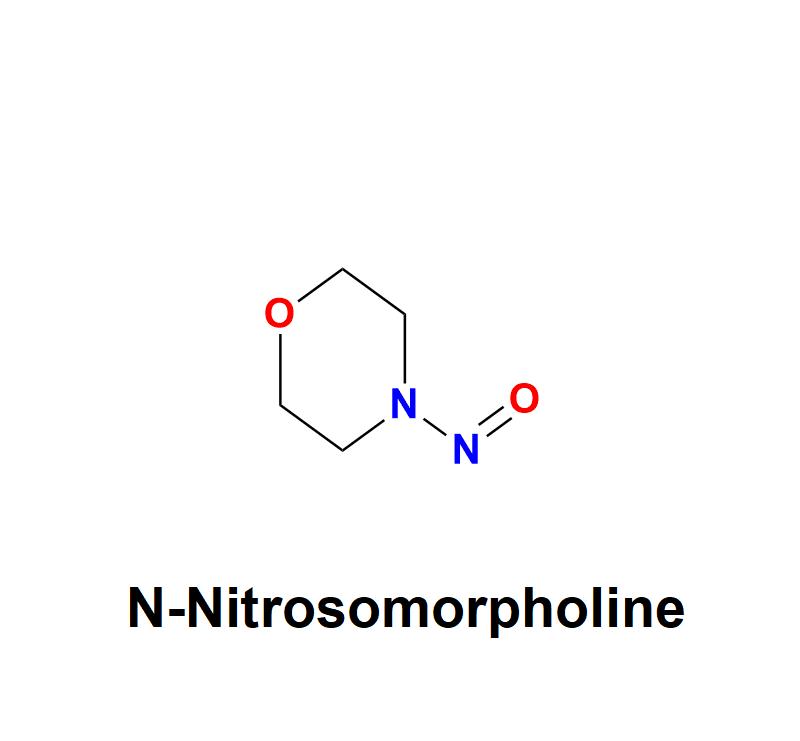
N-Nitrosomorpholine: Addressing Pharmaceutical Safety Challenges with Aquigen Bi …
N-Nitrosomorpholine, a compound belonging to the nitrosamine family, has garnered significant attention in the pharmaceutical and healthcare industries due to its potential carcinogenic risks. This chemical impurity, often found as a byproduct in manufacturing processes, poses serious challenges to drug safety and human health, necessitating stringent monitoring and control measures from pharmaceutical companies.
Learn more about N-Nitrosomorpholine: https://aquigenbio.com/product/n-nitrosomorpholine/
Understanding N-Nitrosomorpholine:
N-Nitrosomorpholine is a nitrosamine impurity characterized by its chemical structure, which includes…
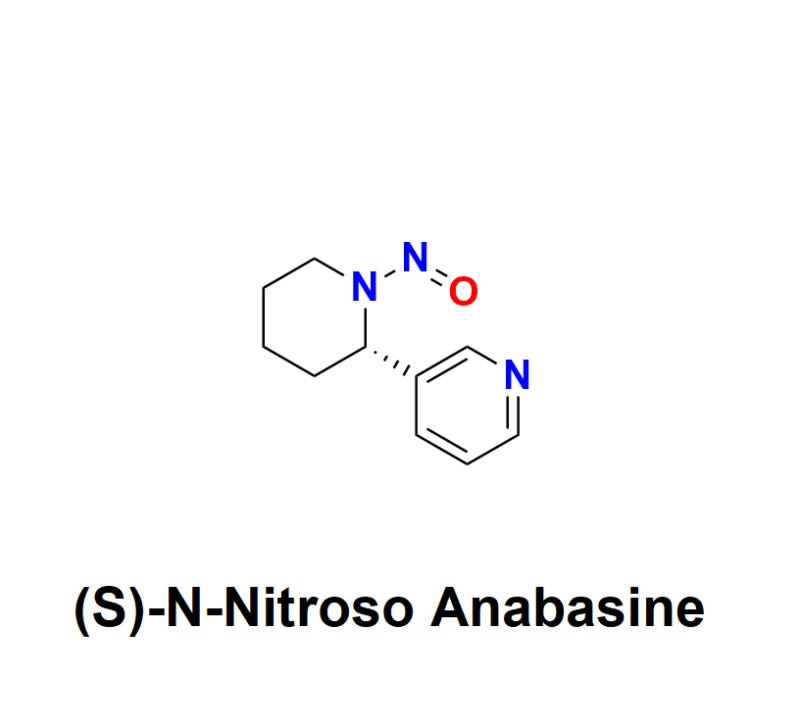
(S)-N-Nitroso Anabasine: Understanding the Risks, Regulatory Challenges, and How …
(S)-N-Nitroso Anabasine, a nitrosamine impurity, has raised significant safety concerns within the pharmaceutical industry. Recognized as a probable human carcinogen, this impurity has become a focal point for global regulatory agencies and manufacturers alike, urging a renewed emphasis on detection, prevention, and management.
Learn more about (S)-N-Nitroso Anabasine: https://aquigenbio.com/product/s-n-nitroso-anabasine/
What Is (S)-N-Nitroso Anabasine?
(S)-N-Nitroso Anabasine belongs to the family of nitrosamines, compounds formed through a chemical reaction known as nitrosation. This…
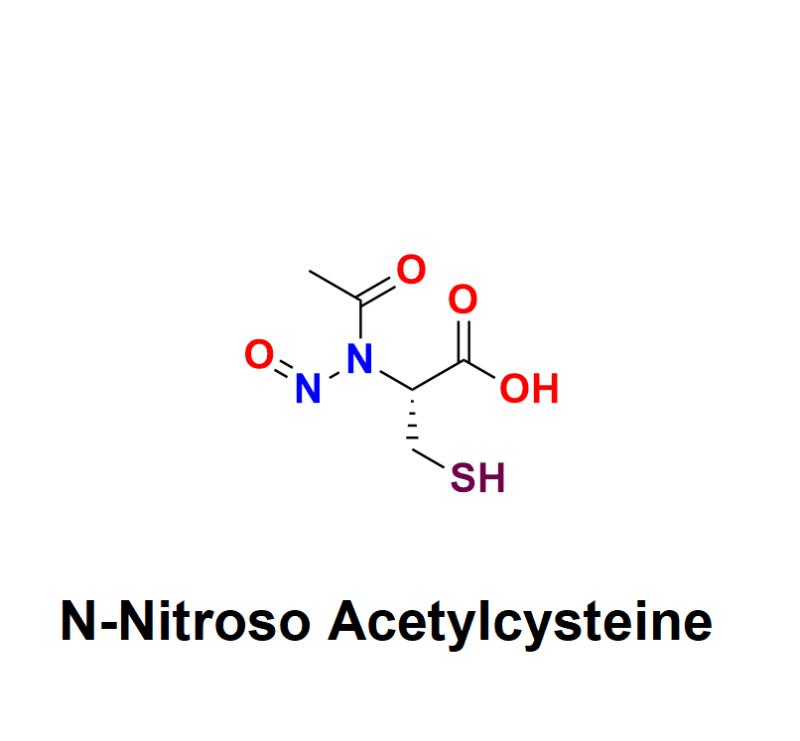
N-Nitroso Acetylcysteine: A Critical Concern in Pharmaceuticals - Exploring Haza …
N-Nitroso Acetylcysteine has emerged as a critical topic of concern in the pharmaceutical industry. As a member of the nitrosamine family, it is a potential impurity that poses significant health risks, including carcinogenicity, even in trace amounts. With increasing regulatory scrutiny on nitrosamine impurities, pharmaceutical manufacturers must address the presence of compounds like N-Nitroso Acetylcysteine to protect public health and ensure compliance with global standards.
Learn more about N-Nitroso Acetylcysteine: https://aquigenbio.com/product/n-nitroso-acetylcysteine/…
More Releases for Elagolix
Endometriosis Treatment Market Size in the 7MM was USD 2,300 million in 2023 and …
DelveInsight's "Endometriosis Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Endometriosis, historical and forecasted epidemiology as well as the Endometriosis market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
Explore Endometriosis Market Trends, treatment landscapes, and emerging therapies shaping the future. Download sample report @ https://www.delveinsight.com/sample-request/endometriosis-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Key Takeaways from the Endometriosis Market Report
• In December 2025, Gesynta Pharma AB conducted…
Endometriosis Market Size is Set for Rapid Growth as Innovative Treatments and R …
The Endometriosis market size is expected to increase during the forecast period with the increasing prevalence, disease awareness, and promising emerging treatment options by leading companies such as Mitsubishi Tanabe Pharma America, Ferring Pharmaceuticals, AbbVie, Neurocrine Biosciences, ObsEva, Kissei Pharmaceuticals, SWK, Enteris BioPharma, Bayer, Hope Medicine, Organon
[Nevada, United States] - DelveInsight's "Endometriosis Market Insights, Epidemiology, and Market Forecast 2034." report offers a detailed examination of Endometriosis, covering historical and predicted…
Orilissa (Elagolix) A Comprehensive Forecast on the Accelerating Market Growth f …
[Las Vegas, United States] DelveInsight, a leader in healthcare research firm, has recently published an in-depth report on Orilissa (Elagolix) AbbVie providing insights into the drug market landscape and market forecast of Orilissa (Elagolix) upto 2032. The report, titled "Orilissa (Elagolix) Market Size, Forecast, and Drug Insight - 2032" is now available for review and analysis.
Are you interested in finding out the projected market size of Orilissa (Elagolix) in…
Pharmaceutical Grade Elagolix Sodium Market: Rising New Business Opportunities f …
Los Angeles, United States,- The research study presented here is a brilliant compilation of different types of analysis of critical aspects of the global Pharmaceutical Grade Elagolix Sodium market. It sheds light on how the global Pharmaceutical Grade Elagolix Sodium market is expected to grow during the course of the forecast period. With SWOT analysis and Porter's Five Forces analysis, it gives a deep explanation of the strengths and weaknesses of…
Pharmaceutical Grade Elagolix Sodium Market Research Report 2023|
QY Research added a new research report to its exhaustive repository. The research report, titled [Pharmaceutical Grade Elagolix Sodium Market], presents an unbiased approach at understanding the market trends and dynamics. Analysts have studied the historical data pertaining to the market and compared it to the current market trends to paint an object picture of the market's trajectory. The report includes a SWOT analysis and Porter's five forces analysis to…
Pharmaceutical Grade Elagolix Sodium Market Analysis, Size and Detail Forecast b …
The global Pharmaceutical Grade Elagolix Sodium market is carefully researched in the report while largely concentrating on top players and their business tactics, geographical expansion, market segments, competitive landscape, manufacturing, and pricing and cost structures. Each section of the research study is specially prepared to explore key aspects of the global Pharmaceutical Grade Elagolix Sodium market. For instance, the market dynamics section digs deep into the drivers, restraints, trends, and…