Press release
N-Nitroso Aniline Research Breakthrough: Pioneering Innovations and Advancements in Chemical Safety Standards to Ensure a Safer Future for Industrial Practices and Environmental Protection
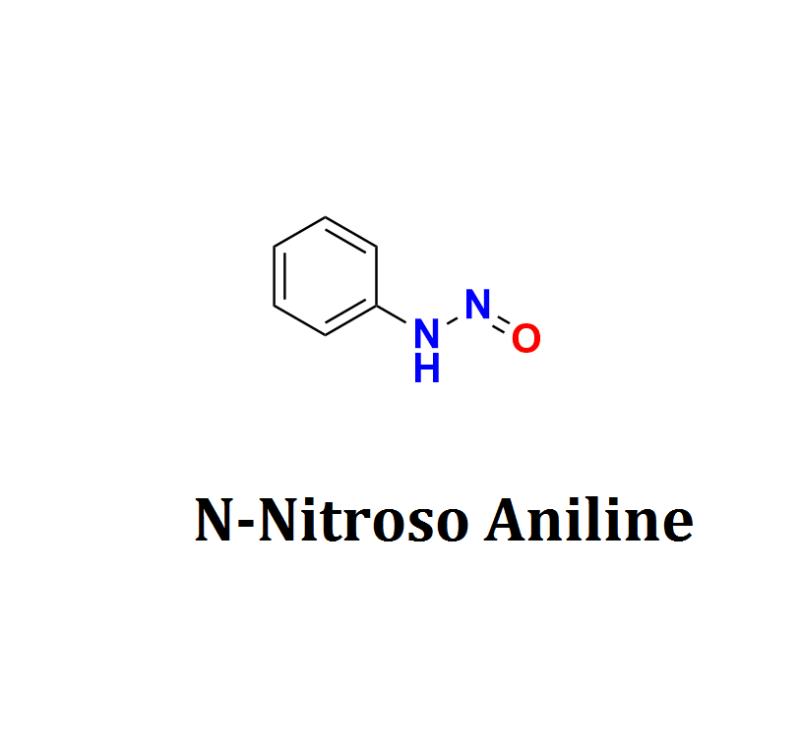
N-Nitroso Aniline, a compound known for its potential carcinogenic properties, is the focus of a significant breakthrough by Aquigen Bio Sciences, a leader in advanced scientific research and contract research services. The company proudly announces a series of groundbreaking developments in the study of N-Nitroso Aniline, addressing critical health and safety concerns associated with this chemical compound and marking a major advancement in chemical safety and public health.N-Nitroso Aniline is a compound formed by the nitrosation of aniline, a widely used industrial chemical involved in dye production, pharmaceuticals, and various other applications. Known for its potential carcinogenic properties, N-Nitroso Aniline poses serious health risks, including a heightened risk of cancer. The need for effective detection, assessment, and mitigation of this compound has never been more urgent.
Access Full Summary - https://aquigenbio.com/product/n-nitroso-aniline/
Aquigen Bio Sciences has made significant strides in addressing these challenges through its latest research initiatives. The company's commitment to improving safety standards and public health is demonstrated through its innovative approaches to detecting, assessing, and mitigating the risks associated with N-Nitroso Aniline.
Advanced Detection Techniques:
One of the standout achievements of Aquigen Bio Sciences is the development of a new, highly accurate detection method for N-Nitroso Aniline. Traditional detection methods for such compounds have often struggled with sensitivity and specificity. To overcome these limitations, Aquigen Bio Sciences has pioneered an advanced approach that combines high-resolution mass spectrometry with cutting-edge chemical assays.
This innovative method allows for the precise identification of even trace amounts of N-Nitroso Aniline in a variety of samples, including industrial effluents, environmental matrices, and consumer products. The enhanced sensitivity and specificity of this technique provide a more reliable tool for regulatory agencies and industries to monitor and manage N-Nitroso Aniline levels, ensuring compliance with safety standards and reducing health risks.
Enhanced Risk Assessment Models:
Understanding the potential health impacts of N-Nitroso Aniline requires sophisticated risk assessment models. Aquigen Bio Sciences has developed and validated new models that offer a more comprehensive evaluation of health risks associated with this compound. These models integrate data from diverse research studies and real-world scenarios to provide a nuanced understanding of how exposure to N-Nitroso Aniline affects human health.
The newly introduced models consider factors such as exposure duration, concentration levels, and individual susceptibility. By incorporating these variables, Aquigen Bio Sciences has created a robust framework that helps predict potential health outcomes with greater accuracy. This enhanced risk assessment capability is a valuable resource for public health officials, policymakers, and researchers working to mitigate the risks associated with N-Nitroso Aniline.
Innovative Mitigation Strategies:
In addition to improving detection and risk assessment, Aquigen Bio Sciences has focused on developing effective strategies to mitigate N-Nitroso Aniline contamination. This includes the creation of advanced filtration systems designed to remove N-Nitroso Aniline from industrial wastewater. These systems employ specialized materials and processes to capture and neutralize harmful compounds before they can enter the environment.
Furthermore, Aquigen Bio Sciences has identified several process modifications that can be implemented in industrial and agricultural settings to minimize the formation of N-Nitroso Aniline. These strategies are aimed at reducing the compound's presence and enhancing safety protocols, contributing to a safer industrial environment and better environmental stewardship.
Get in Touch with Us - https://aquigenbio.com/contact-us/
Collaborative Research Initiatives:
Recognizing the complexity of N-Nitroso Aniline research, Aquigen Bio Sciences has embarked on collaborative research initiatives with leading academic institutions and industry partners. These collaborations are designed to expand research capabilities, share expertise, and accelerate the development of effective solutions.
By working closely with top researchers and scientists, Aquigen Bio Sciences is able to validate findings, explore new research avenues, and refine mitigation strategies. This collaborative approach not only enhances the quality of research but also fosters innovation and knowledge exchange across the scientific community, driving progress in the field of chemical safety.
Public Health Impact:
The advancements in detection, risk assessment, and mitigation strategies developed by Aquigen Bio Sciences are poised to make a significant impact on public health. By addressing the risks associated with N-Nitroso Aniline, the company is contributing to safer industrial practices, improved environmental protection, and enhanced public health outcomes.
Aquigen Bio Sciences' commitment to advancing scientific knowledge and implementing practical solutions underscores its role as a leader in the field of chemical safety. The company's efforts align with global health objectives and support the broader goal of reducing cancer risks and promoting sustainability.
Future Directions:
Looking ahead, Aquigen Bio Sciences plans to continue its research and development efforts in the field of N-Nitroso Aniline. The company is focused on exploring new research areas, improving existing technologies, and addressing emerging challenges. By remaining at the forefront of scientific innovation, Aquigen Bio Sciences aims to drive further advancements in chemical safety and contribute to the well-being of society.
Similar Trending Products:
1. Nitroso Irbesartan 1 - https://aquigenbio.com/product/nitroso-irbesartan-1/
2. (S)-3-(Boc-amino)piperidine - https://aquigenbio.com/product/s-3-boc-aminopiperidine-2/
3. N-Nitroso-D-proline - https://aquigenbio.com/product/n-nitroso-d-proline/
Contact Us:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Us:
Aquigen Bio Sciences is a research organization based in Pune, India, specializing in a wide range of scientific research and development services. With a focus on innovation and excellence, Aquigen Bio Sciences delivers high-quality solutions to meet the complex needs of the pharmaceutical, environmental, and industrial sectors. The company is dedicated to advancing scientific knowledge and improving safety through cutting-edge research and technology.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release N-Nitroso Aniline Research Breakthrough: Pioneering Innovations and Advancements in Chemical Safety Standards to Ensure a Safer Future for Industrial Practices and Environmental Protection here
News-ID: 3662612 • Views: …
More Releases from Aquigen Bio Sciences

Elevate Pharmaceutical R&D with Aquigen BioSciences' Precision‐Grade Flibanser …
Flibanserin Impurity B is a reference standard used in pharmaceutical research and development. It is primarily applied during the analysis and validation of drug substances to identify, quantify, and control impurities that may be present in the final product. This impurity is associated with the parent compound, Flibanserin, a medication approved for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women.
Aquigen BioSciences offers Flibanserin Impurity B as a…

Estradiol Valerate EP Impurity A - Premium Reference Standard for Analytical Dev …
Estradiol Valerate EP Impurity A is a high-quality reference standard designed to meet the stringent requirements of pharmaceutical research, method validation, and quality control processes.
Explore Estradiol Valerate EP Impurity A :
https://aquigenbio.com/product/estradiol-valerate-ep-impurity-a/
Manufactured and characterized with precision, this impurity standard supports laboratories and manufacturers in achieving consistent, reliable, and reproducible results in critical analytical workflows.
With its exceptional purity and accurate characterization, Estradiol Valerate EP Impurity A plays a vital role…

High-Purity N-Nitroso Betahistine D3 for Precise Pharmaceutical Analysis | Deute …
Product Overview
N-Nitroso Betahistine D3 is a premium deuterated nitrosamine impurity standard, specifically developed for precise analytical testing in pharmaceutical laboratories. This reference standard is widely used for analytical method development, validation, and quality control processes to meet stringent regulatory guidelines. With exceptional purity, complete documentation, and reliable traceability, it is ideal for research, development, and compliance applications.
https://aquigenbio.com/product/n-nitroso-betahistine-d3/
Key Features and Benefits
Deuterated Design for Precision: The incorporation of deuterium improves mass spectrometric…

Aquigen Bio Strengthens Pharmaceutical Research with High-Purity Icatibant Impur …
Aquigen Bio, a trusted supplier of pharmaceutical reference standards, today announced the expansion of its Icatibant Impurity Standards portfolio, designed to support drug developers, analytical laboratories, and research organizations with reliable materials for impurity profiling and quality control.
Icatibant, a selective bradykinin B2 receptor antagonist, is widely used in the treatment of hereditary angioedema (HAE). Given its peptide-based structure, Icatibant is prone to the formation of impurities during synthesis and storage.…
More Releases for Nitroso
Riociguat N-Nitroso Des Formyl Impurity - Reliable Reference Standard for Advanc …
Riociguat N-Nitroso Des Formyl Impurity is a premium-quality analytical reference material developed to support research, testing, and quality control in the pharmaceutical industry.
As regulatory guidelines tighten worldwide, accurate detection, quantification, and control of nitroso impurities have become an essential part of drug development and manufacturing.
This compound plays a vital role in ensuring that formulations containing Riociguat meet the highest safety and compliance standards.
The Growing Importance of Nitroso Impurity…
Tizanidine Nitroso Impurity 1 - High-Purity Reference Standard for Reliable Impu …
In the highly regulated world of pharmaceutical manufacturing, impurity profiling plays a critical role in ensuring drug safety, efficacy, and regulatory compliance. Among the wide range of impurity reference standards required during API development and validation, Tizanidine Nitroso Impurity 1 stands out as a vital compound for researchers and manufacturers working on Tizanidine-based formulations.
At Aquigen Bio, we are proud to offer Tizanidine Nitroso Impurity 1, a high-purity reference standard meticulously…
Aquigen Bio Unveils N-Nitroso Felodipine: A New Benchmark in Pharmaceutical Refe …
In an era where drug safety and efficacy are paramount, the pharmaceutical industry faces increasing pressure to develop robust analytical methods that ensure the purity and quality of medicinal products. N-Nitroso Felodipine is specifically engineered to address these challenges. It serves as an indispensable tool for Analytical Method Development (AMD) and Analytical Method Validation (AMV), providing a reliable benchmark against which new and existing analytical procedures can be assessed for…
N-Nitroso Impurities: Carcinogenic Risks, Analytical Challenges, and Compliance …
N-Nitroso impurities are emerging as a significant concern in the pharmaceutical industry due to their potential carcinogenicity. These compounds, formed during the manufacturing or storage of drug products, have raised alarms among regulatory agencies across the globe. Pharmaceutical companies face mounting pressure to identify, analyze, and mitigate these impurities through stringent guidelines to ensure patient safety.
The serious risks posed by N-Nitroso compounds stem from their ability to induce genetic mutations…
Bumetanide Nitroso Impurity: Mitigating Compliance Risks and Integrating Innovat …
Bumetanide Nitroso Impurity has emerged as a significant concern in the pharmaceutical industry, particularly for companies involved in the development and manufacturing of medications used to manage edema. As awareness grows about the potential risks associated with nitroso impurities, regulatory agencies worldwide are increasing scrutiny over pharmaceutical products. This press release aims to address the challenges posed by Bumetanide Nitroso Impurity, outline the regulatory landscape, and highlight innovative practices that…
Sumatriptan Nitroso Impurity: Navigating Compliance Challenges and Implementing …
Sumatriptan Nitroso Impurity has become a pressing concern in the pharmaceutical industry, as companies grapple with significant compliance challenges related to these potentially harmful contaminants. As Sumatriptan, a widely prescribed medication for migraine relief, comes under increasing scrutiny from regulatory bodies, pharmaceutical companies must navigate a complex landscape to ensure product safety and adhere to evolving regulatory standards.
What is the issue?
Nitroso impurities have emerged as a pressing concern within the…