Press release
Huateng Pharma Develops Intermediates of Carfilzomib For Multiple Myeloma
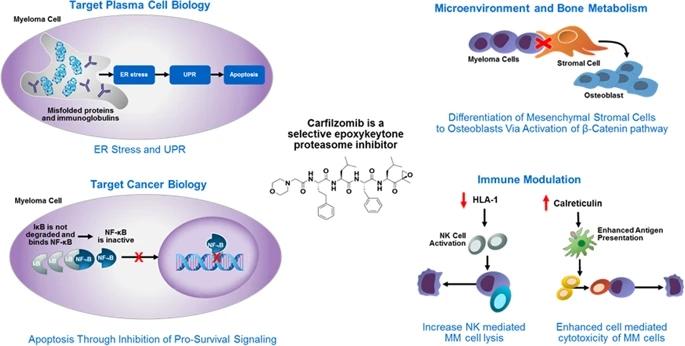
Carfilzomib, a proteasome inhibitor, is indicated for the treatment of patients with multiple myeloma.Multiple myeloma (multiple myeloma, MM) is a malignant tumor originating from the B cell lineage, characterized by the clonal proliferation of malignant plasma cells in the bone marrow microenvironment, causing fractures and bone marrow failure, and is the second most common blood system disease in the world Tumors cannot be cured by traditional chemotherapy regimens.
Bortezomib is the first proteasome inhibitor and the first-line drug for multiple myeloma, which has made a breakthrough in the treatment of multiple myeloma. Due to its strong drug resistance and people's resistance to it Continuous research on drug mechanism, carfilzomib was approved by the FDA as the second proteasome inhibitor after bortezomib, and received at least 2 drugs before treatment (including bortezomib and immunomodulators) ) patients with multiple myeloma. Carfilzomib is a specific, irreversible targeted inhibitor originally developed by Proteolix, produced by Onyx Pharmaceuticals, and approved by the FDA on July 20, 2012.
(1)Mechanism of action of carfilzomib
Carfilzomib binds irreversibly to the chylase of the 20S proteasome, whereas bortezomil binds reversibly. In addition, carfilzomib also inhibits trypsin and semi-acyl-like aspartase. Irreversible inhibition and selective inhibition confer a potential advantage over bortezomib in both efficacy and tolerability. Carfilzomib's structure and mechanism of action are different from that of bortezomib, the dipeptide boric acid analogous. Bortezomib reversibly binds to the catalytic β5 subgroup of the proteasome, while the catalytic β5 subgroup and the immune proteasome β5i(LMP7) subgroup of cafezomib irreversibly covalently binds to the proteasome, which have better potency and resistance compared to bortezomib.
(2) Safety and Tolerability
Compared with bortezomib, carfilzomib had less peripheral neuropathy. In RRMM patients, the rate of grade 3 peripheral neuropathy was 1.3%, but no patients had grade 4 events, and only 1% of patients required dose adjustment or Withdrawal. Long-term treatment with carfilzomib did not increase the risk of psychosis.
(3) Drug resistance
About 60% of patients will eventually develop resistance to bortezomib, and the average time for drug resistance to appear is within one year of bortezomib administration. Clinical studies have shown that carfilzomib is safe to replace bortezomib and can effectively treat MM patients who failed bortezomib treatment.
Huateng Pharma is worldwide known for a variety of pharmaceutical intermediates used in research and development. We can provide intermediates of Carfilzomib such as (S)-methyl 2-((S)-2-tert.butoxycarbonyIamino-4-phenylbutanamido-4-methylpentanamido)-3-phenylpropanoate (CAS NO.868539-96-2), tert-Butyl ((s)-4-methyl-1-((r)-2-methyloxiran-2-yl)-1-oxopentan-2-yl)carbamate (CAS NO.247068-82-2), 1-Pentanone,2-amino-4-methyl-1-[(2R)-2-methyl-2-oxiranyl]-,(2S)-,2,2,2-trifluoroacetate(1:1) (CAS NO.247068-85-5), and Morpholin-4-ylacetic acid (CAS NO.3235-69-6). We have our own factory and make scale-up production with capacities varying from gram to kilograms and multi tons.
Company: Hunan HuaTeng Pharmaceutical Co., Ltd.
Address: Lugu Business Plaza E1, Yuelu District, Changsha City, Hunan Province, P.R. China.
ZIP Code: 410006
Email: sales@huatengusa.com
Website: https://en.huatengsci.com/
Huateng Pharma is a leading and professional manufacturer which can provide pharmaceutical intermediates, PEG derivatives, biochemical reagents, APIs, Vitamin D Derivatives and so on. We have a 34,000 square meter manufacturing site with advanced design concept of intelligent manufacturing, which can realize the integration of the production process and accomplish the complete transformation of lab scale - pilot plant - large-scale commercial production, with an annual capacity of more than 1 billion RMB.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Huateng Pharma Develops Intermediates of Carfilzomib For Multiple Myeloma here
News-ID: 2822861 • Views: …
More Releases from Hunan Huateng Pharmaceutical Co. Ltd.

Huateng Pharma Supplies Minoxidil Intermediate 2,4-Diamino-6-chloropyrimidine (C …
Minoxidil was first introduced by Upjohn Company of the United States, and was first used as an oral drug for the treatment of refractory hypertension in the 1970s. In later clinical applications, doctors observed hair regrowth and generalized excessive hair in balding patients, which led to the development of minoxidil preparations.
Minoxidil can increase local blood supply, stimulate the proliferation and differentiation of hair follicle epithelial cells, so as to promote…

Huateng Pharma Supplies Anti-diabetic API Intermediates With Huge Stock
Diabetes is a serious chronic disease characterized by elevated blood sugar concentrations associated with the effects of abnormal beta cell biology on insulin action. Diabetes occurs when the pancreas does not produce enough insulin or the body does not use the insulin it does produce efficiently.
The most common forms of diabetes are type 1 diabetes and type 2 diabetes. Type 1 diabetes is characterized by insufficient insulin production and…
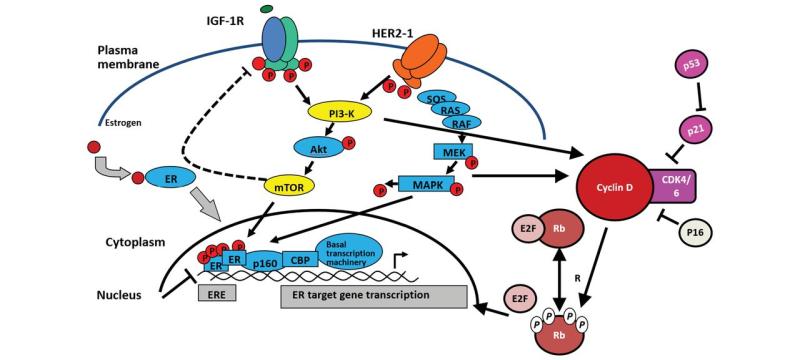
Huateng Pharma Supplies Some Intermediates of CDK4/6 Inhibitors for Treatment of …
Breast cancer is the most frequent cancer among women, impacting 2.1 million women each year, and also causes the greatest number of cancer-related deaths among women. In 2018, it is estimated that 627,000 women died from breast cancer - that is approximately 15% of all cancer deaths among women. While breast cancer rates are higher among women in more developed regions, rates are increasing in nearly every region globally.
The majority…
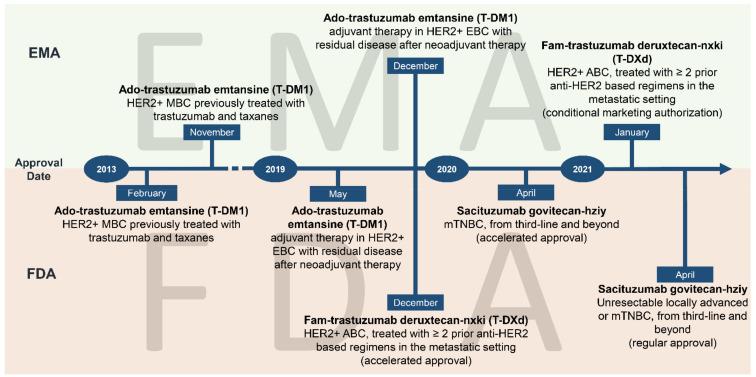
ADC Drugs For HER2 Positive Breast Cancer
According to the latest data, breast cancer has overtaken lung cancer to become the most common cancer among women, and the death rate is the second highest among female tumors, seriously affecting the physical and mental health of women around the world. Patients with abnormal expression of human epidermal growth factor receptor (HER2) account for 15%-20% of all breast cancers, which is highly invasive and has poor prognosis.
Although more drug…
More Releases for Carfilzomib
Carfilzomib API market: Factors Benefitting Emergence of New Entrants | Dr. Redd …
"The Carfilzomib API global market is thoroughly researched in this report, noting important aspects like market competition, global and regional growth, market segmentation and market structure. The report author analysts have estimated the size of the global market in terms of value and volume using the latest research tools and techniques. The report also includes estimates for market share, revenue, production, consumption, gross profit margin, CAGR, and other key factors.…
Kyprolis (carfilzomib) Market Expected to Secure Notable Revenue Share During 20 …
Kyprolis (Carfilzomib) is an anti-cancer medicine that inhibits the growth and spread of cancer cells in the body. Kyprolis is sometimes used with other drugs such as dexamethasone or lenalidomide and administered by intravenous infusion to treat patients with blood cancer or multiple myeloma that affect plasma cells. Kyprolis is a second-generation proteasome inhibitor that irreversibly binds to the threonine-containing active site at the N-terminus of the 20S proteasome (the…
Kyprolis (carfilzomib) Market to Witness Growth Acceleration by Top Key Players …
The Global Kyprolis (carfilzomib) Market size was valued at USD XX Million in 2020 and is predicted to reach USD XX Million by 2030 with a CAGR of XX% from 2021-2030.
Kyprolis (Carfilzomib) is an anti-cancer medicine that inhibits the growth and spread of cancer cells in the body. Kyprolis is sometimes used with other drugs such as dexamethasone or lenalidomide and administered by intravenous infusion to treat patients with blood…
Carfilzomib Market || Research Report, Growth Forecast 2020-2030
This research study on "Carfilzomib market" reports offers the comparative assessment of Carfilzomib market and consist of Historical data, Significance, statistical data, size & share, Market Price & Demand, Business overview, Market Analysis By Product and Market Trends by Key Players. This Carfilzomib Market is Segmented in two type on the basis of type of materials and end-users. It has global market covered in all the regions, ranging to that…
Carfilzomib Market by Top Players – Amgen Inc., Vijayasri Organics Limited, Te …
Carfilzomib Market By Product Type (Type I and Type II); and End-User (Anti-Cancer and Others); - Global Industry Analysis And Forecast To 2025
Industry Outlook
Carfilzomib is abbreviated as CFZ. Carfilzomib ia an anti-cancer drug used for blocking proteasome cellular complexes that break down protein. Carfilzomib is developed by Onyx Pharmaceuticals and sold under the name Kyprolis. Carfilzomib is used for treatment of people suffering from multiple myeloma (cancer associated to…
Trends in Carfilzomib Market: Business Analysis 2018-2025 by SWOT analysis, Shar …
InForGrowth has added a new research article on the healthcare industry category. The report titled " Global Carfilzomib Market Research Report 2018" can be used to gain important insight into this market. Report users can add data from this report to their knowledge of the global market in order to lay a solid foundation for successful business and investment choices in this market. The report provides a professional, progressive, and…