Press release
Huateng Pharma Supplies Intermeidates of Pemetrexed Against NSCLC
Pemetrexed disodium is a drug successfully developed by Eli Lilly in the United States for the treatment of tumors, patent protection to January 2017. Pemetrexed disodium is a multi-targeted anti-folate agent with a core pyrrolopyrimidine moiety in its structure. It is a dual inhibitor of nucleotide synthase/dihydrofolate reductase, which inhibits cell replication by disrupting the normal process of folate-dependent metabolism in cells and simultaneously blocking three different enzyme targets critical for the survival of cancer cells, thereby inhibiting tumor growth.In February 2004, the U.S. Food and Drug Administration (FDA) approved pemetrexed disodium in combination with cisplatin to treat a rare cancer, malignant pleural mesothelioma. Malignant pleural mesothelioma is a rare lung pleural cancer. Pemetrexed disodium can interfere with cancer cell replication and further promote tumor cell apoptosis by blocking the unique mechanism of 3 key enzymes of cancer cell metabolism.
This treatment plan is a synergistic inhibitory effect of three targets, which is superior to the single-target treatment plan using cisplatin in the past. At the same time, by supplementing folic acid and vitamins, the toxic and side effects can be effectively controlled and the patient's tolerance can be enhanced. In August 2004, the FDA approved pemetrexed disodium as a second-line treatment for locally advanced lung cancer or metastatic non-small cell lung cancer with fast approval. In December 2005, pemetrexed disodium, a patented drug of Eli Lilly, was launched in China and was approved for the treatment of malignant pleural mesothelioma.
According to the statistics of the world's best-selling drugs, the sales of pemetrexed in 2005 were 463 million US dollars, and in 2014 it reached a peak of 2.792 billion US dollars. Sales in 2017 were $2.063 billion. Its global sales declined after 2015 due to patent expiry.
In December 2005, Eli Lilly's pemetrexed disodium was launched in China. With the addition of domestic imitation products, the market competition was fierce.
In the first three quarters of 2021, domestic sales of pemetrexed disodium reached 3.679 billion, a year-on-year increase of 4.19%. The drug sales market is crowded by generic drug companies, accounting for 78.49%. However, affected by volume purchases, only original research drugs and products that have passed the consistency evaluation of generic drugs can enter the centralized procurement process. Therefore, Sichuan Huiyu Pharma and Eli Lilly are more competitive than other companies, accounting for 42.55% and 21.51% respectively.
Huateng Pharma, as a leading pharmaceutical intermediates supplier in China, can provide CAS NO.137281-39-1, CAS NO.165049-28-5, CAS NO.1118-89-4, CAS NO.155405-80-4 and CAS NO.3140-73-6 from lab to commercial scale, which can be used as Pemetrexed disodium intermediates. Also, we can provide CDMO services to meet your requirements, combining a high level of competencies, equipment and quality.
Address: Lugu Business Plaza E1, Yuelu District, Changsha City, Hunan Province, China
Manufacturing Base: Tongguan Kiln, Wangcheng district, Changsha, Hunan, China
Email: sales@huatengusa.com
Website: https://us.huatengsci.com/
Zip code: 410205
Telephone: +86 731 89916275
Fax: +86 0731-82251112-818
Huateng Pharma is a global provider in contract development and manufacturing for intermediates. With our focus on operational excellence, and the integration of manufacturing operations with EHS, quality assurance, quality control, regulatory, supply chain management and project management, we can manufacture, scale-up and supply high-quality intermediates and APIs from our China facility.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Huateng Pharma Supplies Intermeidates of Pemetrexed Against NSCLC here
News-ID: 2662737 • Views: …
More Releases from Hunan Huateng Pharmaceutical Co. Ltd.

Huateng Pharma Supplies Minoxidil Intermediate 2,4-Diamino-6-chloropyrimidine (C …
Minoxidil was first introduced by Upjohn Company of the United States, and was first used as an oral drug for the treatment of refractory hypertension in the 1970s. In later clinical applications, doctors observed hair regrowth and generalized excessive hair in balding patients, which led to the development of minoxidil preparations.
Minoxidil can increase local blood supply, stimulate the proliferation and differentiation of hair follicle epithelial cells, so as to promote…

Huateng Pharma Supplies Anti-diabetic API Intermediates With Huge Stock
Diabetes is a serious chronic disease characterized by elevated blood sugar concentrations associated with the effects of abnormal beta cell biology on insulin action. Diabetes occurs when the pancreas does not produce enough insulin or the body does not use the insulin it does produce efficiently.
The most common forms of diabetes are type 1 diabetes and type 2 diabetes. Type 1 diabetes is characterized by insufficient insulin production and…
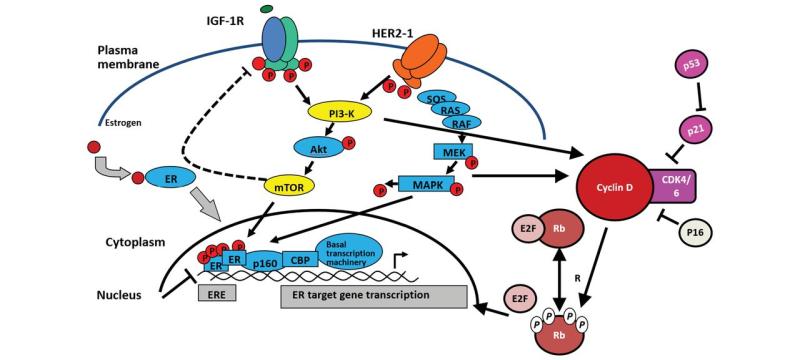
Huateng Pharma Supplies Some Intermediates of CDK4/6 Inhibitors for Treatment of …
Breast cancer is the most frequent cancer among women, impacting 2.1 million women each year, and also causes the greatest number of cancer-related deaths among women. In 2018, it is estimated that 627,000 women died from breast cancer - that is approximately 15% of all cancer deaths among women. While breast cancer rates are higher among women in more developed regions, rates are increasing in nearly every region globally.
The majority…
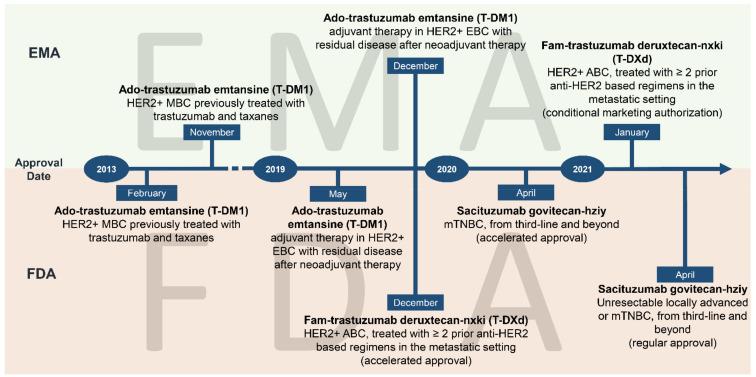
ADC Drugs For HER2 Positive Breast Cancer
According to the latest data, breast cancer has overtaken lung cancer to become the most common cancer among women, and the death rate is the second highest among female tumors, seriously affecting the physical and mental health of women around the world. Patients with abnormal expression of human epidermal growth factor receptor (HER2) account for 15%-20% of all breast cancers, which is highly invasive and has poor prognosis.
Although more drug…
More Releases for Pemetrexed
Pemetrexed Injection Market Key Players, Share and Forecast Outlook
"The Pemetrexed Injection Market Is Set To Grow At An Estimated CAGR Of 7.5% From 2025 To 2034, Rising From $1.2 Billion In 2024 To $2.5 Billion By 2034."
On May 15, 2025, Exactitude Consultancy., Ltd. released a research report titled "Pemetrexed Injection Market". This report covers the global Pemetrexed Injection market sales, sales volume, price, market share, ranking of major companies, etc., and provides a detailed analysis by region, country,…
Pemetrexed Market Share, Growth, And Forecast to 2033 Cadila, Abbott, Eli Lilly
Pemetrexed is a chemotherapy drug primarily used to treat non-small cell lung cancer (NSCLC) and malignant pleural mesothelioma. It functions as an antifolate, inhibiting enzymes involved in the synthesis of purines and pyrimidines, which are necessary for DNA and RNA production. This disruption prevents cancer cell proliferation. Pemetrexed is typically given in combination with other chemotherapy agents, like cisplatin, and is administered through an intravenous infusion. It can cause side…
Pemetrexed Market Development Trends & Competitive Analysis by Leading Industry …
Allied Market Research added new research on Pemetrexed Market: Global Opportunity Analysis and Industry Forecast, 2022-2029.
The Pemetrexed Market explores comprehensive study on various segments like size, share, development, innovation, sales and overall growth of major players. The research is based on primary and secondary data sources and it consists both qualitative and quantitative detailing.
Request The Free Sample PDF Of This Report:
https://www.alliedmarketresearch.com/request-sample/10728
Which market perspectives are enlightened in the Pemetrexed Market report?
Executive…
Pemetrexed Disodium API Market Size, Share, Development by 2025
LP INFORMATION recently released a research report on the Pemetrexed Disodium API market analysis and elaborate the industry coverage, current market competitive status, and market outlook and forecast by 2025. Moreover, it categorizes the global Pemetrexed Disodium APImarket by key players, product type, applications and regions,etc.
The main objective of this market research is to help the readers understand the structure of Pemetrexed Disodium APImarket, market definition, overview, industry opportunities…
Pemetrexed Market | Eli Lilly and Company, Stada Arzneimittel AG, Eagle Pharmace …
The Pemetrexed market demand is anticipated to flourish during the forecast period 2020-2027. The report offers information related to import and export, along with the current business chain in the market at the global level. This report provides an in-depth overview of the Pemetrexed market. This includes market characteristics, consisting of segmentation, market share, trends and strategies for this market. The Market Size section provides historical forecasts of market growth…
Pemetrexed Disodium API Market Size, Share, Development by 2024
Global Info Research offers a latest published report on Pemetrexed Disodium API Market Analysis and Forecast 2019-2025 delivering key insights and providing a competitive advantage to clients through a detailed report. This report focuses on the key global Pemetrexed Disodium API players, to define, describe and analyze the value, market share, market competition landscape, SWOT analysis and development plans in next few years.
To analyze the Pemetrexed Disodium API with respect…
