Press release
Urinary Tract Infection Treatment Market Foreseen to Witness 3% CAGR During 2019 - 2027
The global urinary tract infection treatment market is projected to amass a large amount of revenues in the coming years. The healthcare industry has shown a deft level of earnestness in dealing with infectious diseases and disorders.Read Report Overview - https://www.transparencymarketresearch.com/urinary-tract-infection-treatment-market.html
Besides, availability of improved research facilities has helped medical scientists in diagnosing the various reasons responsible for urinary tract infection. It is safe to expect that the global urinary tract infection treatment market would become a safe haven for investment in the years to follow. Rapid advancements in the field of nephrology have also played a part in driving market demand.
Transparency Market Research (TMR), in one of its reports, predicts that the global urinary tract infection treatment market would expand at a sluggish CAGR of 3% over the period between 2019 and 2027. Furthermore, the total market value is expected to touch US$ 7.5 Bn in 2027, growing from a value of US$ 5.9 Bn in 2018.
Request Brochure of Report - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=72872
The slow rate of growth can be attributed to the delays in approval of several drugs. However, the presence of a seamless healthcare industry shall continue to complement market growth. Moreover, new research initiatives pertaining to medicine and healthcare shall also drive market demand.
Approvals from FDA as a Forerunner to Market Growth
The Food and Drug Administration (FDA) has been the harbinger of multiple improvements in the healthcare industry. As the need for improved treatment mechanisms gathers momentum across the world, FDA is playing a defining role in modern medicine. In its latest, FDA approved the use of FETROJA® (cefiderocol) drug for the treatment of urinary tract infections.
Request for Analysis of COVID-19 Impact on Urinary Tract Infection Treatment Market - https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=72872
The occurrence of certain complicated forms of urinary tract infections in adults could be deadly. These types of infections do not have an alternative line of treatment, except the use of heavy drugs. FDA's new approval is expected to aid the growth of the global urinary tract infection treatment market.
Availability of Improved Medical Facilities to Drive Demand
The adverse effects of insufficient water consumption are widely discussed by medical practitioners and doctors. Furthermore, urinary infections are often an outcome of low water consumption. Therefore, medications for treatment of urinary tract infection are widely distributed across hospitals and pharmacies. Therefore, the global urinary tract infection treatment market is set to undergo revolutionary growth in the years to follow.
Enquiry Before Buying - https://www.transparencymarketresearch.com/sample/sample.php?flag=EB&rep_id=72872
The advent of better healthcare facilities within nephrology have played a vital role in driving market demand. The geriatric population is highly vulnerable to the incidence of urinary tract infection. Therefore, improvements in geriatric care have also given a thrust to market growth.
Some of the leading vendors operating in the global urinary tract infection market are AstraZeneca plc, Pfizer, Inc., Johnson & Johnson, F. Hoffmann La-Roche Ltd., Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim GmbH, Novartis AG.
More Trending Reports by Transparency Market Research -
Digital Dose Inhalers Market: https://www.prnewswire.com/news-releases/global-digital-dose-inhalers-market-to-reach-a-value-of-us-4-3-bn-by-2027--growing-at-a-cagr-of-10-from-2019-to-2027-transparency-market-research-300999357.html
Drugs of Abuse Testing Market: https://www.prnewswire.com/news-releases/high-intake-of-illegal-drugs-particularly-amongst-the-young-generation-to-bolster-growth-of-the-global-drugs-of-abuse-testing-market-low-income-countries-to-offer-plentiful-growth-opportunities-for-the-market-participants-tmr-301252768.html
Contact Us
Mr. Rohit Bhisey
Transparency Market Research,
90 State Street, Suite 700,
Albany, NY 12207
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: https://www.transparencymarketresearch.com/
About Us
Transparency Market Research is a global market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants, use proprietary data sources and various tools and techniques to gather, and analyse information. Now avail flexible Research Subscriptions, and access Research multi-format through downloadable databooks, infographics, charts, interactive playbook for data visualization and full reports through MarketNgage, the unified market intelligence engine. Sign Up for a 7 day free trial!
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Urinary Tract Infection Treatment Market Foreseen to Witness 3% CAGR During 2019 - 2027 here
News-ID: 2560627 • Views: …
More Releases from Transparency Market Research
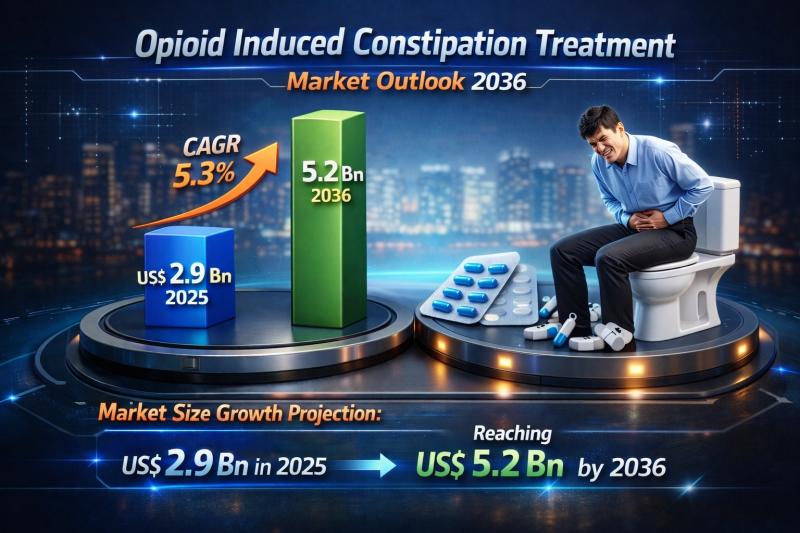
Global Opioid Induced Constipation Treatment Market Set to Reach USD 5.2 Billion …
The global opioid induced constipation (OIC) treatment market is witnessing steady and sustained growth as healthcare systems worldwide place increasing emphasis on comprehensive pain management and supportive care. Valued at US$ 2.9 billion in 2025, the market is projected to reach US$ 5.2 billion by 2036, expanding at a compound annual growth rate (CAGR) of 5.3% from 2026 to 2036. Growth is primarily fueled by the rising prevalence of chronic…
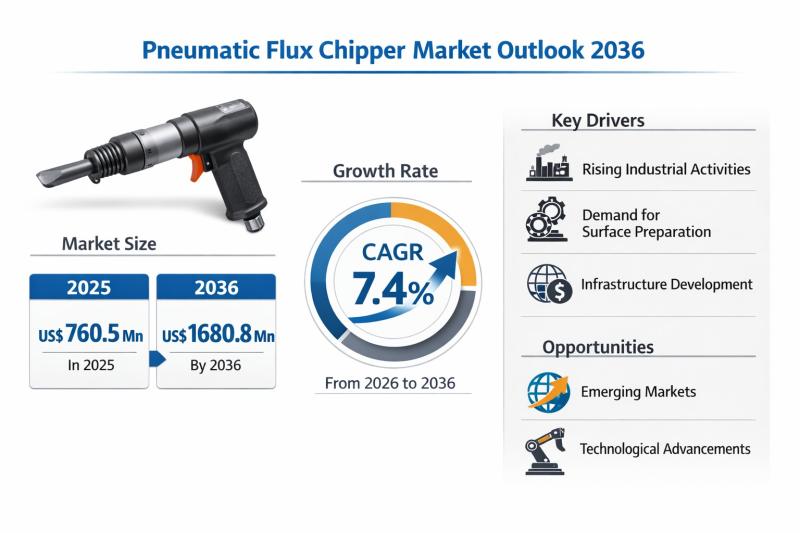
Pneumatic Flux Chipper Market Expanding at 7.4% CAGR Through 2036 - By Product T …
The global Pneumatic Flux Chipper Market is set to witness sustained and resilient growth over the next decade, underpinned by expanding heavy manufacturing activities, rising welding and fabrication demand, and continuous investments in industrial infrastructure across emerging and developed economies. According to the latest industry analysis, the market was valued at US$ 760.5 Mn in 2025 and is projected to reach US$ 1,680.8 Mn by 2036, expanding at a compound…

AI in Automotive Market Outlook 2036: Global Industry to Surge from US$ 19.8 Bil …
The AI in automotive market is entering a phase of exponential expansion, supported by rapid digitization of vehicles, growing safety mandates, and consumer demand for intelligent mobility. The global market was valued at US$ 19.8 Bn in 2025 and is projected to reach US$ 244.4 Bn by 2036, registering a remarkable CAGR of 27% from 2026 to 2036.
This growth trajectory reflects the transition of automobiles from mechanically driven products to…
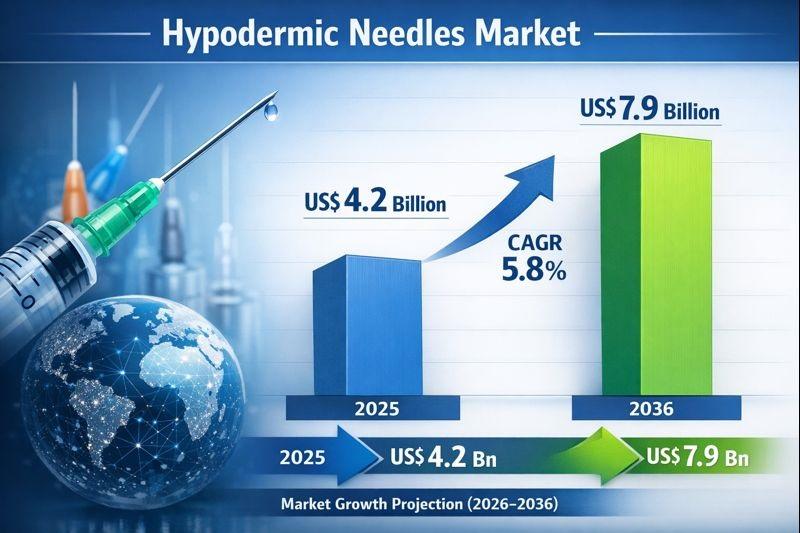
Hypodermic Needles Market to Reach US$ 7.9 Billion by 2036 on Rising Injectable …
The global hypodermic needles market was valued at approximately US$ 4.2 billion in 2025 and is projected to reach around US$ 7.9 billion by 2036, expanding at a CAGR of nearly 5.8% from 2026 to 2036, driven by the rising prevalence of diabetes, cancer, and chronic diseases, growing demand for injectable drugs and biologics, and the expansion of global vaccination and immunization programs; increasing adoption of safety-engineered and disposable needles,…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
