Press release
Stem Cells in new spinal cord paraplegia study showing improvements
MD Stem Cells reports initial results of their new study in paraplegia using Bone Marrow Derived Stem Cells (BMSC) have shown a prompt improvement in sensory function. The Stem Cell Spinal Cord Injury Exoskeleton and Virtual Reality (SciExVR) study is an Institutional Review Board approved, patient sponsored clinical study registered with the National Institutes of Health (NIH) NCT 03225625. It uses a multi-injection and application approach of BMSC for patients with Thoracic and Lumbar spinal cord injuries or disease with motor, sensory and/or bowel-bladder dysfunction. In addition to the BMSC applications, arms include exoskeleton use and virtual reality use to further stimulate the motor and sensory neurons of the brain.“We are excited that at the 1 month post- SciExVR visit our initial patient showed recovery of neurologic sensation - from 1 to 2 spinal cord segments improved from the original loss of function level. This was on the American Spinal Injury Association (ASIA) Injury Assessment (AIA) measured by the patient’s own physician at his rehabilitation facility pre and post-treatment” indicated Dr. Steven Levy MD, CEO of MD Stem Cells and Study Director for the SciExVR study. “It is likely that the paraspinal multi-injection of BMSC at, above and below the spinal cord injury level, combined with BMSC given intravenously and intranasally, is positively affecting the sensory cells in the dorsal roots and their associated ascending fibers”.
The American Spinal Injury Association is the leading organization in spinal cord injury and care in North America. The ASIA Injury Assessment is a comprehensive, reproducible test of sensory functions such as light touch and pin prick, as well as motor function meaning strength and movement of muscles. The exact level of impairment can be determined and any changes noted over time.
In the SciExVR study mesenchymal and other stem cells are separated from the bone marrow using an FDA cleared medical device and in full compliance with FDA guidelines on use of these stem cells. The procedure is performed in a fully licensed surgical center with board certified anesthesiology support by a fellowship trained spinal surgeon. In addition to the application of the stem cells in separate paraspinal locations, intravenous and intranasal- arms 2 and 3 are designed to potentially increase motor neuron stimulation with the hope this will encourage neuronal transdifferentiation of the BMSC. Arm 2 allows patients to use motorized external support for walking which is called an Exoskeleton and is increasingly available at rehabilitation facilities across the country. Arm 3 uses Virtual Reality exposure to similarly stimulate motor and sensory cells.
MD Stem Cells believes improvements in motor function may take at least a year to occur. Early neuroglial cell reformation, exosomal release of neurotrophic factors and mitochondrial transfer may offer initial motor recovery in neurons that are still partially viable. The ultimate goal is reconnection of transected or injuried upper motor neurons to the lower motor neurons through bridging neurons. The belief is that the brain will relearn and adjust control of any new motor and sensory reconnections which may not match the original pre-injury pathways.
“There is certainly evidence in the scientific literature of BMSC undergoing neuronal transdifferentiation in the Central Nervous System (CNS). In our own study in ophthalmology called the Stem Cell Ophthalmology Treatment Study (SCOTS) we have evidence of CD34 marked bone marrow stem cells transdiffentiating into NeuN+ cells which are neurons” remarked Dr. Levy. “We are cautiously optimistic that the same can occur along the length of the spinal cord and benefit paraplegia.”
Patients with thoracic or lumber spinal cord disease or injuries may contact Dr. Levy for more information on the SciExVR study. stevenlevy@mdstemcells.com 203-423-9494.
MD Stem Cells is a trusted and valued source of the latest information regarding clinically available adult stem cell treatments for patients. MD Stem Cells coordinates patient referrals and manages the treatment process for patients and providers.
MD Stem Cells
Steven Levy MD, CEO
3 Sylvan Road South
Westport, CT 06880
stevenlevy@mdstemcells.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Stem Cells in new spinal cord paraplegia study showing improvements here
News-ID: 832509 • Views: …
More Releases from MD Stem Cells
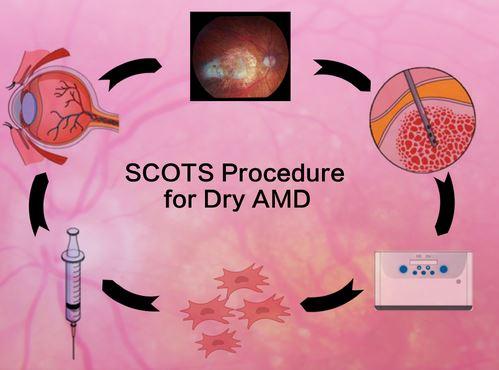
New treatment shows vision benefit in Age Related Macular Degeneration
MD Stem Cells reports dry AMD results from their clinical study SCOTS- the Stem Cell Ophthalmology Treatment Study. 63% of treated and evaluated eyes achieved improvement in vision while an additional 34% had vision remain stable in follow up. No complications occurred.
==============================================================================
Age Related Macular Degeneration- specifically dry AMD- will affect almost 200 million people world-wide in 2020. An approach, pioneered by MD Stem Cells, using bone marrow stem…

Dr. Steven Levy, CEO of MD Stem Cells, joins editorial board of Clinical Trials …
MD Stem Cells announces that the company’s President and CEO, Steven Levy MD, has joined the editorial board of Clinical Trials in Degenerative Diseases (CTDD). In addition to his role as executive officer for MD Stem Cells, Dr. Levy is Study Director for several pivotal stem cell clinical trials using autologous bone marrow derived stem cells. These including SCOTS –the Stem Cell Ophthalmology Treatment Study (NCT 0192086) ,…
More Releases for Cell
Cell Sorting Market Accelerates as Cell Therapy, Immuno-Oncology & Single-Cell R …
The rising focus on precision medicine, immunotherapy, and advanced cell-based research is driving the global cell sorting market into a high-growth phase. With expanding applications in stem cell therapy, CAR-T manufacturing, cancer immunology, and single-cell genomics, demand for accurate, high-purity cell isolation systems is stronger than ever. This release highlights key market trends, segmentation insights, technological innovations, and the factors shaping the future of cell sorting.
Download Full PDF Sample Copy…
Cell Isolation Cell Separation Market Size Analysis by Application, Type, and Re …
According to Market Research Intellect, the global Cell Isolation Cell Separation market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The market for cell isolation and separation is expanding rapidly as a result of sophisticated biotechnological…
Cell Free Protein Synthesis Market Beyond the Cell: Revolutionizing Protein Prod …
Cell-Free Protein Synthesis Market to reach over USD 457.13 Mn by the year 2031 - Exclusive Report by InsightAce Analytic
"Cell-Free Protein Synthesis Market" in terms of revenue was estimated to be worth $265.94 Mn in 2023 and is poised to reach $457.13 Mn by 2031, growing at a CAGR of 7.20% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for free Sample Pages: https://www.insightaceanalytic.com/request-sample/1445
Current…
Cell Expansion Market - Expand the Boundaries of Cell Therapy: Redefine Cell Exp …
Newark, New Castle, USA: The "Cell Expansion Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cell Expansion Market: https://www.growthplusreports.com/report/cell-expansion-market/7939
This latest report researches the industry structure, sales, revenue,…
Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect …
Researchmoz added Most up-to-date research on "Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect Cell and Others" to its huge collection of research reports.
This report researches the worldwide GMP Cell Banking market size (value, capacity, production and consumption) in key regions like North America, Europe, Asia Pacific (China, Japan) and other regions.
This study categorizes the global GMP Cell Banking breakdown data by manufacturers, region, type…
Cell Culture Market Size, Cell Culture Market Share, Cell Culture Market Trends …
According to a new research published by Polaris Market Research the global cell culture market is anticipated to reach more than USD 49 billion by 2026. Cell culture is a rapidly emerging as an implement for analyzing and treating various disease such as Alzheimer’s and cancer.
Request for Sample of This Research Report @ https://bit.ly/2D7pZ5u
Top Key Players: -
Becton,
Dickinson and Company
Biospherix
EMD Millipore
Eppendorf AG
Merck KGaA
Sartorius AG
VWR International
Cell culture is a rapidly emerging…
