Press release
New treatment shows vision benefit in Age Related Macular Degeneration
MD Stem Cells reports dry AMD results from their clinical study SCOTS- the Stem Cell Ophthalmology Treatment Study. 63% of treated and evaluated eyes achieved improvement in vision while an additional 34% had vision remain stable in follow up. No complications occurred.==============================================================================
Age Related Macular Degeneration- specifically dry AMD- will affect almost 200 million people world-wide in 2020. An approach, pioneered by MD Stem Cells, using bone marrow stem cells from the actual patient has now shown visual improvement for 63% of dry AMD eyes and stability in another 34%, demonstrating statistical significance in helping patients with this blinding disease. Results were recently published in the Medicines Journal- a highly regarded international medical journal.
Title of the paper is: Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Age-Related Macular Degeneration.
There is no FDA approved medication for treating dry AMD. Certain vitamins may reduce the risk of bleeding or wet AMD. But vitamins do not appear to stop the relentless loss of retinal cells and vision called geographic atrophy (GA) from AMD.
The highlight of the MD Stem Cell report was that 63% of dry AMD eyes treated and followed had vision improvement. This ranged from 2.5% to 44.6% with an average of 27.6% on a scientific vision scale called LogMAR. An additional 34% of eyes remained stable for the follow up period- important because many of the eyes had previously been losing vision. The findings were highly statistically significant with p < 0.001 meaning that the results overwhelmingly met that medical standard and confirming that the BMSC treatment was responsible for the improvements seen.
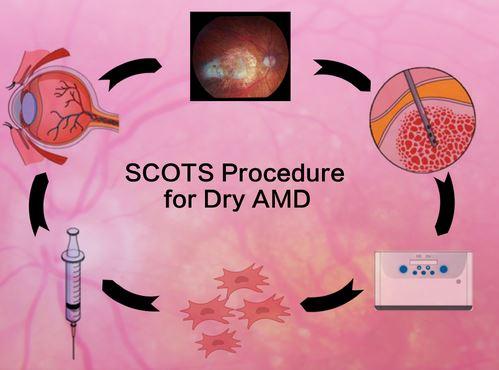
The Stem Cell Treatment Ophthalmology Study- both SCOTS and SCOTS2- has been treating many different eye diseases since 2013 using the patient's own bone marrow stem cells (BMSC) injected in the orbit around the eye. The study is Institutional Board Approved and National Institutes of Health registered on the government ClinicalTrials website NCT 03011541. The physicians involved with MD Stem Cells now have 14 world class medical and scientific publications primarily reporting their clinical results in ophthalmology. This is vastly more than any other stem cell research group working with eye disease and should be reassuring to patients and health care providers seeking treatment options. Different optic nerve diseases, NAION, LHON, DOA, optic atrophy; as well as several retinal diseases including Retinitis Pigmentosa, Ushers and now AMD have all shown benefit. MD Stem Cells has worked to achieve the safest, most effective approach to dry AMD using BMSC - with gratifying success.
"Following multiple patient treatments and over a dozen peer-reviewed papers, our studies have shown that a patient's own bone marrow stem cells (BMSC) can have positive effects on different retinal and optic nerve diseases" explains Dr. Levy, CEO and Chief Science Officer for MD Stem Cells. "As we have continued the study, other researchers have published numerous papers revealing how this may be occurring: release of exosomes with neurotrophic factors helping neurons and photoreceptors, transfer of cytoplasmic structures such as mitochondria to injured cells, and transdifferentiation of BMSC into neurons."
Dr. Levy concludes: "The research has shown that patients with dry AMD choosing to participate in the SCOTS 2 may have a significant likelihood of either improving or stabilizing their vision. "
Patients may receive information about SCOTS 2 by emailing , using the contact us page on the website , or calling 203-423-9494. The Stem Cell Ophthalmology Study 2 is enrolling patients with different retina and optic nerve diseases. MD Stem Cells has no grant support and is not a pharmaceutical company; these are patient sponsored studies and the patients pay for both treatment and travel.
Steven Levy MD
MD Stem Cells
3 Sylvan Road South
Westport, CT 06880 USA
stevenlevy@mdstemcells.com
MD Stem Cells is a trusted and valued source of the latest information regarding clinically available adult stem cell treatments for patients. Many previously chronic and progressive diseases are able to be treated with a patients own stem cells. With many available options it can be confusing making a choice. MD Stem Cells provides education and information so patients can make the best decisions for themselves and their family members. MD Stem Cells coordinates patient referrals and manages the pre-treatment process for patients and providers. MD Stem Cells develops and manages stem cell practices globally.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release New treatment shows vision benefit in Age Related Macular Degeneration here
News-ID: 2014139 • Views: …
More Releases from MD Stem Cells

Stem Cells in new spinal cord paraplegia study showing improvements
MD Stem Cells reports initial results of their new study in paraplegia using Bone Marrow Derived Stem Cells (BMSC) have shown a prompt improvement in sensory function. The Stem Cell Spinal Cord Injury Exoskeleton and Virtual Reality (SciExVR) study is an Institutional Review Board approved, patient sponsored clinical study registered with the National Institutes of Health (NIH) NCT 03225625. It uses a multi-injection and application approach of…

Dr. Steven Levy, CEO of MD Stem Cells, joins editorial board of Clinical Trials …
MD Stem Cells announces that the company’s President and CEO, Steven Levy MD, has joined the editorial board of Clinical Trials in Degenerative Diseases (CTDD). In addition to his role as executive officer for MD Stem Cells, Dr. Levy is Study Director for several pivotal stem cell clinical trials using autologous bone marrow derived stem cells. These including SCOTS –the Stem Cell Ophthalmology Treatment Study (NCT 0192086) ,…
More Releases for Cell
Cell Sorting Market Accelerates as Cell Therapy, Immuno-Oncology & Single-Cell R …
The rising focus on precision medicine, immunotherapy, and advanced cell-based research is driving the global cell sorting market into a high-growth phase. With expanding applications in stem cell therapy, CAR-T manufacturing, cancer immunology, and single-cell genomics, demand for accurate, high-purity cell isolation systems is stronger than ever. This release highlights key market trends, segmentation insights, technological innovations, and the factors shaping the future of cell sorting.
Download Full PDF Sample Copy…
Cell Isolation Cell Separation Market Size Analysis by Application, Type, and Re …
According to Market Research Intellect, the global Cell Isolation Cell Separation market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The market for cell isolation and separation is expanding rapidly as a result of sophisticated biotechnological…
Cell Free Protein Synthesis Market Beyond the Cell: Revolutionizing Protein Prod …
Cell-Free Protein Synthesis Market to reach over USD 457.13 Mn by the year 2031 - Exclusive Report by InsightAce Analytic
"Cell-Free Protein Synthesis Market" in terms of revenue was estimated to be worth $265.94 Mn in 2023 and is poised to reach $457.13 Mn by 2031, growing at a CAGR of 7.20% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for free Sample Pages: https://www.insightaceanalytic.com/request-sample/1445
Current…
Cell Expansion Market - Expand the Boundaries of Cell Therapy: Redefine Cell Exp …
Newark, New Castle, USA: The "Cell Expansion Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cell Expansion Market: https://www.growthplusreports.com/report/cell-expansion-market/7939
This latest report researches the industry structure, sales, revenue,…
Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect …
Researchmoz added Most up-to-date research on "Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect Cell and Others" to its huge collection of research reports.
This report researches the worldwide GMP Cell Banking market size (value, capacity, production and consumption) in key regions like North America, Europe, Asia Pacific (China, Japan) and other regions.
This study categorizes the global GMP Cell Banking breakdown data by manufacturers, region, type…
Cell Culture Market Size, Cell Culture Market Share, Cell Culture Market Trends …
According to a new research published by Polaris Market Research the global cell culture market is anticipated to reach more than USD 49 billion by 2026. Cell culture is a rapidly emerging as an implement for analyzing and treating various disease such as Alzheimer’s and cancer.
Request for Sample of This Research Report @ https://bit.ly/2D7pZ5u
Top Key Players: -
Becton,
Dickinson and Company
Biospherix
EMD Millipore
Eppendorf AG
Merck KGaA
Sartorius AG
VWR International
Cell culture is a rapidly emerging…