Press release
Virtual Clinical Trials Market to Reach USD 14.42 Billion by 2033 | Strong 5.5% CAGR | North America Leads with 40% Share | Key Players: IQVIA, ICON, Labcorp, Science 37, Parexel
Market OverviewThe Global Virtual Clinical Trials Market reached US$ 8.95 billion in 2024 and is projected to grow to US$ 14.42 billion by 2033, expanding at a CAGR of 5.5% during the forecast period 2025-2033. The market has shown significant growth over recent years, rising from US$ 4.60 billion in 2022 to US$ 4.95 billion in 2023, reflecting the increasing adoption of decentralized clinical research. Virtual clinical trials, also known as decentralized clinical trials (DCTs), are remotely conducted studies that leverage digital technologies such as mobile apps, wearables, and telemedicine platforms to collect patient data, monitor participants, and manage trial procedures without requiring physical visits to clinical sites. These trials offer a patient-centric and flexible approach, removing barriers such as travel time and logistical challenges, which are common in traditional clinical trials. The growing demand for virtual trials is driven by several factors, including improved patient convenience, higher recruitment and retention rates, cost efficiency, and broader access to diverse patient populations. Platforms like Science 37 have demonstrated the effectiveness of virtual trials by enabling participation from remote or underserved areas, resulting in significant growth in enrollment and adherence. With increasing technological adoption and regulatory support, virtual clinical trials are expected to continue transforming the clinical research landscape, making it more accessible, efficient, and patient-friendly.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/virtual-clinical-trials-market?Juli
Recent Developments:
✅ January 2026: Lokavant launched Spectrum v15, a forecasting platform optimized for hybrid and decentralized trial environments, enhancing predictive planning and operational efficiency during study execution.
✅ December 2025: Medable partnered with Google Cloud and Masimo to integrate medical-grade wearables and cloud scalability into decentralized clinical trial workflows, enabling real-time remote monitoring and stronger data capture across global sites.
✅ November 2025: Lindus Health collaborated with the Tiefenbacher Group to run virtual trials for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), highlighting adoption of virtual trial models across new therapeutic areas.
✅ September 2025: Nordic life-science organizations launched the Nordic Virtual Office, streamlining access to regional research networks and expanding virtual and hybrid clinical trial capabilities across Europe.
✅ June 2025: PicnicHealth introduced Clinical Services with Virtual Clinic and Virtual Site offerings, improving observational research and remote patient management for life sciences companies.
Mergers & Acquisitions:
✅ January 2026: eMed, LLC acquired Science 37, a leading decentralized clinical trials platform, enhancing decentralized trial enrollment and remote patient access.
✅ December 2025: Lindus Health raised $55 million in Series B funding led by Balderton Capital to scale its AI-enabled eClinical platform for faster, more efficient virtual trials.
✅ November 2025: Suvoda merged with Greenphire to create an integrated clinical trial technology stack, covering patient randomization, payments, and decentralized trial operations.
✅ September 2025: Science 37 expanded its partnerships with pharmaceutical sponsors to implement multi-site virtual clinical trials, strengthening remote patient engagement and operational capacity.
✅ July 2025: Medable secured strategic funding and partnerships to integrate AI-driven analytics into its decentralized trial platform, improving trial monitoring, adherence, and data quality.
Buy Now & Unlock 360° Market Intelligence:-https://www.datamintelligence.com/buy-now-page?report=virtual-clinical-trials-market?Juli
Key Players:
IQVIA | ICON plc | Laboratory Corporation of America Holdings (Labcorp) | Science 37 | Parexel International Corporation | Oracle Corporation | Medidata Solutions | Signant Health | Veristat, LLC | Sanofi
Key Highlights:
IQVIA - Holds a significant market presence, driven by its global contract research services, real-world data analytics, and decentralized trial solutions enabling remote patient monitoring and hybrid trial models.
ICON plc - Supported by hybrid and fully virtual clinical trial services across multiple therapeutic areas, improving patient recruitment and trial efficiency.
Laboratory Corporation of America Holdings (Labcorp) - Provides centralized testing and decentralized trial support, enhancing remote data collection and monitoring capabilities.
Science 37 - Fueled by its MetaSiteTM decentralized trial platform, enabling home-based participation, remote data capture, and broader patient access.
Parexel International Corporation - Driven by its virtual and hybrid clinical trial management solutions and regulatory expertise across global studies.
Oracle Corporation - Supports cloud-based clinical trial management and eClinical solutions for virtual and decentralized trials.
Medidata Solutions (Dassault Systèmes) - Focused on technology platforms for virtual trial execution, including eCOA, eConsent, and data analytics.
Signant Health - Powered by digital health solutions for remote patient monitoring, eCOA, and eConsent to optimize decentralized trial operations.
Veristat, LLC - Provides full-service CRO support with virtual trial operations, patient engagement, and regulatory compliance services.
Sanofi - Pharmaceutical sponsor increasingly adopting decentralized trial models, leveraging virtual trials to accelerate clinical research and patient access.
Market Segmentation:
➥By study design, Interventional Trials account for approximately 55% of the market, driven by remote administration and digital monitoring of therapies; Observational Trials contribute around 35%, focusing on virtual data collection without assigned interventions; and Expanded Access Trials make up about 10%, providing investigational treatments to patients outside traditional trial protocols.
➥By indication, Oncology dominates with an estimated 28% share, reflecting high adoption of decentralized platforms for cancer studies, followed by Neurology at 15%, Autoimmune/Inflammation at 12%, Cardiovascular Disease at 10%, Metabolic/Endocrinology at 8%, Infectious Disease at 7%, Ophthalmology at 5%, and Other indications such as rare and pediatric diseases comprising 15% of the market.
➥By trial phase, Phase III trials hold the largest share at 40%, benefiting from broad patient recruitment and remote monitoring capabilities, followed by Phase II at 25%, Phase I at 20%, and Phase IV post-marketing studies at 15%, leveraging virtual tools for real-world evidence collection and patient follow-ups.
Speak to Our Analyst and Get Customization in the report as per your requirements:-https://www.datamintelligence.com/customize/virtual-clinical-trials-market?Juli
Regional Insights:
North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America dominates the market with an estimated 40% share, driven by the presence of leading CROs, strong adoption of digital health technologies, and favorable regulatory frameworks supporting decentralized trials.
Europe holds around 25%, supported by robust clinical research infrastructure, government incentives for decentralized studies, and growing adoption of hybrid trial models. The Asia-Pacific region accounts for approximately 20%, reflecting expanding clinical trial activities, increasing investment in digital health platforms, and a large patient population for virtual recruitment.
Latin America contributes about 10%, driven by rising CRO activity and growing interest in remote patient monitoring, while the Middle East & Africa represents roughly 5%, with emerging virtual trial initiatives and gradually improving healthcare infrastructure. The market's regional growth is fueled by increasing regulatory acceptance of decentralized models, technological adoption, and the need for efficient patient recruitment across diverse geographies.
Market Dynamics:
The growth of the virtual clinical trials market is being significantly driven by improved data accuracy and real-time monitoring. Virtual trials enable continuous tracking of vital signs, symptoms, and medication adherence, providing more accurate and timely patient data compared to periodic in-person visits. Platforms such as Medable equip patients with wearables that monitor physiological parameters like heart rate, oxygen levels, and physical activity, transmitting data in real time to researchers. This allows for early detection of adverse events, faster decision-making, and enhanced trial reliability. Real-time monitoring also promotes patient engagement and adherence, as digital tools can remind participants to take medications or complete trial activities, especially in long-duration studies. For example, in June 2024, Materna Medical launched a virtual clinical trial evaluating the effectiveness of the Milli Vaginal Dilator in treating vaginismus, demonstrating how virtual trials enable novel data collection while maintaining patient convenience.
On the other hand, the market faces notable restraints related to data privacy and security concerns. Virtual clinical trials rely heavily on the collection, storage, and transmission of sensitive patient data, including medical histories and health metrics, which makes them vulnerable to breaches, unauthorized access, and non-compliance with data protection regulations. Cybersecurity incidents, such as the Colorado-based pathology laboratory breach affecting over 1.8 million patients, highlight the risks associated with digital health platforms. Concerns over privacy and the potential misuse of personal health data can reduce patient trust, lower enrollment rates, and negatively impact retention, ultimately hindering the effectiveness of decentralized clinical trials. According to the HHS Cybersecurity Program, over 630 healthcare organizational breaches affected nearly 29 million healthcare records, emphasizing the critical need for robust security measures to support virtual trial adoption.
📌 Request for 2 Days FREE Trial Access: https://www.datamintelligence.com/reports-subscription
☛ Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
☛ Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg?Juli
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Virtual Clinical Trials Market to Reach USD 14.42 Billion by 2033 | Strong 5.5% CAGR | North America Leads with 40% Share | Key Players: IQVIA, ICON, Labcorp, Science 37, Parexel here
News-ID: 4342164 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
United States Immunohistochemistry Market 2026 | Growth Drivers, Trends & Market …
Market Size and Growth
The Global Immunohistochemistry Market size was valued at US$ 2,302.79 million in 2022 and is estimated to reach US$4,240.65 million by 2031, growing at a CAGR of 8.1% during the forecast period (2024-2031).
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/immunohistochemistry-market?sb
Key Development:
United States: Recent Industry Developments
✅ In February 2026, the U.S. Food and Drug Administration approved PD‐L1 IHC 22C3 pharmDx…
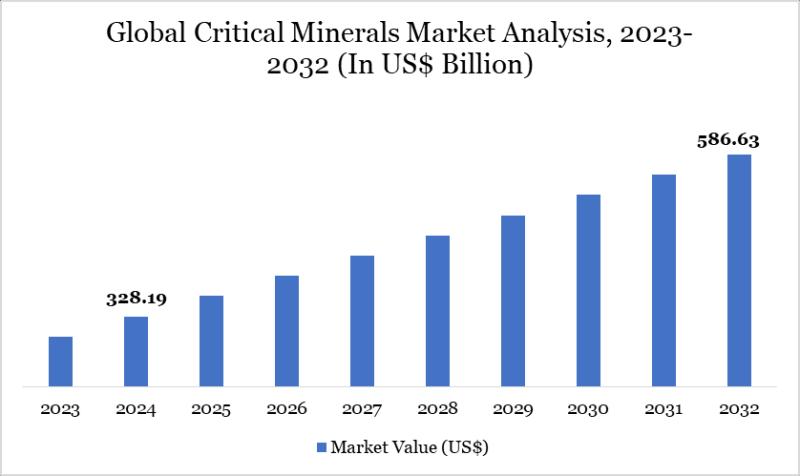
Critical Minerals Market to Reach US$ 586.63 Billion by 2032; Asia Pacific Leads …
Market Overview
Market Size (2024): USD 328.19 billion
Expected Market Size (2032): USD 586.63 billion
CAGR (2025-2032): 7.53%
The global critical minerals market is experiencing unprecedented growth, driven primarily by the accelerating transition to clean energy technologies. Key energy transition minerals including lithium, cobalt, and nickel have seen rapid demand growth: lithium demand has tripled, cobalt demand has risen by 70%, and nickel demand has increased by 40% between 2017 and 2022. Clean energy…
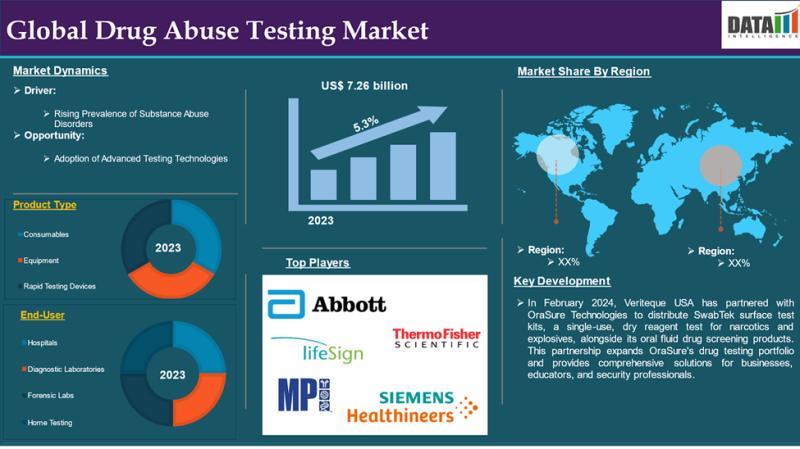
Drug of Abuse Testing Market to Reach US$ 12.31 Billion by 2033; North America L …
Market Overview
Market Size (2023): USD 7.26 billion
Expected Market Size (2033): USD 12.31 billion
CAGR (2025-2033): 5.3%
Drug abuse testing is a critical process for detecting prescription and illicit substances in an individual's system, ensuring safety, regulatory compliance, and medical evaluation. It is widely used across workplace screening, forensic investigations, sports anti-doping programs, and clinical diagnostics.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/drug-of-abuse-testing-market?Juli
Advancements in rapid detection…
United States Aesthetic Lasers and Energy Devices Market 2026 | Growth Drivers, …
Market Size and Growth
Aesthetic Lasers and Energy Devices Market is estimated to reach at a CAGR of 6.40% during the forecast period (2024-2031).
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/aesthetic-lasers-and-energy-devices-market?sb
Key Development:
United States: Recent Industry Developments
✅ In November 2025, Venus Concept Inc. secured FDA 510(k) clearance for its Venus NOVATM platform, a next‐generation multi‐application device combining radiofrequency, EMS, and electromagnetic technologies for non‐invasive…
More Releases for Virtual
Virtual Office Staff and Fortitude Virtual Professionals Unite to Elevate Virtua …
On May 5, 2025, two esteemed virtual assistant agencies-Virtual Office Staff and Fortitude Virtual Professionals-officially merged, forming a dynamic alliance poised to redefine the standards of virtual executive support and business operations.
This strategic merger combines the strengths of both organizations, uniting their commitment to delivering top-tier virtual office services that exceed client expectations. Operating under the Fortitude Virtual Professionals brand, the unified entity leverages a robust infrastructure and a reputation…
Virtual Assistance Revolution: Intelligent Virtual Assistants Market (2023-2032)
Global Intelligent Virtual Assistants Market Scope and Overview Report 2023-2032
"According to the research report, the global intelligent virtual assistant market was valued at USD 2.62 billion in 2022 and is expected to reach USD 22.45 billion by 2032, to grow at a CAGR of 24.0% during the forecast period."
This visionary report entitled Intelligent Virtual Assistants Market: By Size, Latest Trends, Share, Huge Growth, Segments, Analysis and Forecast, 2030 published by…
Virtual Workplace Market Seeking Excellent Growth | Opus Virtual Offices, Blackm …
Advance Market Analytics published a new research publication on "Virtual Workplace Market Insights, to 2027" with 232 pages and enriched with self-explained Tables and charts in presentable format. In the Study you will find new evolving Trends, Drivers, Restraints, Opportunities generated by targeting market associated stakeholders. The growth of the Virtual Workplace market was mainly driven by the increasing R&D spending across the world.
Get Free Exclusive PDF Sample Copy of…
Virtual Internet launches “On-Demand” Virtual Data Centers
LONDON, England Feb. 21, 2011 | Virtual Internet – Virtual Internet has announced a new cloud package called Virtual Data Centers which allow IT managers to virtualize their infrastructure “on-demand” via the Internet.
The new self-service package allows managers to slice and dice a minimum of 10GB (or more) of physical disk space and/or physical memory into multiple configurations at will.
Incorporating the best features of a private cloud and Infrastructure-as-a-Service (IaaS),…
Caribbean Association Of Virtual Assistants Open Virtual Assistant Directory To …
CAVA Seeks Outside Assistance In Order To Meet Increased Demands For Virtual Assistants
The Caribbean Association Of Virtual Assistants, the Caribbean’s leading association for virtual assistants is set to accept listings from virtual assistants the world over.
The industry is still fairly new in the Caribbean and due to ever-increasing demands for the services provided by members of the association, the directors have made the decision to seek outside assistance.
Virtual assistants…
Virtual Assistants Become a Little Less Virtual
Toronto, Canada … North American Virtual Assistants and particularly Canadian VA’s have never before had the opportunity to meet and exchange views in this kind of forum and on Canadian soil. Barb Lang, a Toronto based Virtual Assistant specializing in Event Planning, saw a need for new and seasoned VA’s to meet in person in order to exchange ideas and business expertise in a mastermind / workshop type event.…
