Press release
European Orthobiologics Market Outlook 2025-2035: Key Developments and Future Scope
The orthobiologics market is set for sustained, healthy growth through 2035 as aging populations, rising rates of musculoskeletal disorders, expanding sports-medicine activity, and innovation in biologic therapies converge. Orthobiologics-biologic materials used to help injuries heal more quickly and effectively (including bone graft substitutes, platelet-rich plasma (PRP), stem cell therapies, growth factors such as BMPs, and scaffolds)-are increasingly replacing or augmenting traditional surgical options by promoting tissue regeneration, speeding recovery, and improving functional outcomes.Surgeons, hospitals, and outpatient orthopaedic clinics are adopting orthobiologics to reduce operative time, lower complication rates, and support minimally invasive procedures. As evidence from clinical trials and real-world registries accumulates, payers and health systems are responding with evolving reimbursement and care-pathway models that further enable adoption-especially in high-volume indications like spinal fusion, fracture repair, arthroscopy, and joint preservation.
Quick Stats (2025-2035) - Estimated:
Market Value 2025 (estimate): USD 7.8 billion
Market Forecast Value 2035 (estimate): USD 14.6 billion
Absolute Growth (2025-2035): ≈ USD 6.8 billion
Forecast CAGR (2025-2035): ≈ 6.4%
Leading Product Segment (2025): Bone graft substitutes & synthetic scaffolds
Fastest-Growing Segment: Cell-based therapies and combination biologic + scaffold products
Key Growth Regions: North America, Europe, Asia-Pacific
To Access the Complete Data Tables & in-depth Insights, Request a Discount on this report: https://www.factmr.com/connectus/sample?flag=S&rep_id=12345
Market Drivers
Aging Population & Osteoarthritis Burden
Demographic aging increases incidence of degenerative joint disease and fragility fractures, fueling demand for regenerative options that restore function and delay or avoid joint replacement surgery. Orthobiologics offering cartilage repair, bone regeneration, and soft-tissue healing align well with this need.
Rising Sports Injuries & Active Lifestyles
Growth in recreational and professional sports participation worldwide increases soft-tissue injuries (tendons, ligaments) and focal cartilage defects. Athletes and active adults seek treatments that shorten downtime and optimize return-to-play-boosting PRP, stem-cell, and scaffold use in orthopaedic practice.
Evidence Generation & Clinical Adoption
Stronger clinical data demonstrating improved healing rates, reduced reoperation, and better functional outcomes for select indications is persuading clinicians to integrate orthobiologics into standard practice. As comparative effectiveness studies accumulate, uptake accelerates for well-validated products.
Minimally Invasive Procedures & Outpatient Care
The shift toward arthroscopic, percutaneous, and outpatient procedures favors biologic adjuncts that can be delivered with lower invasiveness. This trend reduces hospitalization costs and supports broader use of orthobiologics in ambulatory settings.
Innovation in Product Complexity
Next-generation orthobiologics-combining scaffolds with growth factors, using engineered cell populations, or offering off-the-shelf allograft scaffolds-are expanding indications and creating premium products with higher per-procedure value.
Market Structure & Segment Insights
Product Types:
Bone Graft Substitutes & Scaffolds: Remain the largest revenue source due to broad use in spinal fusion, trauma, and joint reconstruction.
Autologous Biologics (PRP, Bone Marrow Concentrate): Widely used for tendon injuries, cartilage lesions, and soft-tissue healing; favored for low regulatory hurdles and point-of-care convenience.
Allogeneic Cell Therapies & Engineered Products: Fastest evolving but face higher regulatory and cost barriers; represent significant long-term upside if clinical benefits and reimbursement are secured.
Growth Factors & Biosynthetic Peptides: Important adjuncts for targeted regenerative responses.
End-Users:
Hospitals, orthopedic specialty centers, ambulatory surgery centers, and sports-medicine clinics drive adoption-each with differing purchasing dynamics and procedural volumes.
Geography:
North America leads due to advanced reimbursement, high procedure volumes, and strong clinical R&D. Europe follows with rising adoption; Asia-Pacific is the fastest growth region as surgical volumes expand and local manufacturing scales.
Challenges & Restraints
Reimbursement & Cost Pressure
High per-procedure cost for advanced biologics and variability in payer coverage limit uptake in some markets. Clear demonstration of cost-effectiveness versus standard care is increasingly essential.
Regulatory Complexity
Cell-based and engineered biologics face stringent regulatory pathways; securing approvals and demonstrating long-term safety slows commercialization and raises development costs.
Clinical Heterogeneity & Evidence Gaps
Mixed trial results and variability in biologic preparations (e.g., PRP composition) create performance uncertainty. Standardization of protocols and high-quality randomized trials are needed to broaden guideline endorsements.
Manufacturing & Supply Chain Constraints
Scale-up for cell therapies and complex scaffolds requires specialized manufacturing, cold-chain logistics, and quality systems-barriers for smaller innovators.
Opportunities & Strategic Directions
Combination Products & Customized Solutions
Integrating biologics with tailored scaffolds, 3D-printed implants, and patient-matched solutions can command premium pricing and clinical preference.
Point-of-Care Systems & Standardization
Point-of-care devices that produce standardized biologic preparations (controlled PRP/BMC systems) improve reproducibility and clinician confidence.
Value-Based Contracting & Outcomes Data
Outcomes-linked reimbursement, bundled payment models, and real-world evidence programs can accelerate access by aligning cost with clinical benefit.
Emerging Market Penetration
Lower-cost autologous therapies and simplified scaffold systems suit emerging markets where surgical volumes are rising.
Outlook:
The orthobiologics market is transitioning from niche adjuncts to mainstream components of musculoskeletal care. With an expected multi-billion-dollar expansion through 2035, growth will be driven by aging demographics, sports-medicine demand, and continued product innovation. Manufacturers that combine strong clinical evidence, scalable manufacturing, reimbursement strategies, and clinician training programs will capture the most value as orthobiologics reshape regenerative musculoskeletal care in the coming decade.
Browse Full Report: https://www.factmr.com/report/orthobiologics-market
Purchase Full Report for Detailed Insights
For access to full forecasts, regional break-outs, product- and application-level analysis, company share details, and emerging trend assessments, you can purchase the complete report: https://www.factmr.com/checkout/12345
Have specific requirements or need assistance on report pricing or have a limited budget? Please contact sales@factmr.com
Related Reports:
Antibiotics Market: https://www.factmr.com/report/antibiotics-market
Infection Control Market: https://www.factmr.com/report/infection-control-market
Immunohistochemistry Market: https://www.factmr.com/report/immunohistochemistry-market
Animal Osteoarthritis Market: https://www.factmr.com/report/animal-osteoarthritis-market
Contact:
US Sales Office
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583, +353-1-4434-232
Email: sales@factmr.com
About Fact.MR:
Fact.MR is a global market research and consulting firm, trusted by Fortune 500 companies and emerging businesses for reliable insights and strategic intelligence. With a presence across the U.S., UK, India, and Dubai, we deliver data-driven research and tailored consulting solutions across 30+ industries and 1,000+ markets. Backed by deep expertise and advanced analytics, Fact.MR helps organizations uncover opportunities, reduce risks, and make informed decisions for sustainable growth.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release European Orthobiologics Market Outlook 2025-2035: Key Developments and Future Scope here
News-ID: 4304912 • Views: …
More Releases from Fact.MR
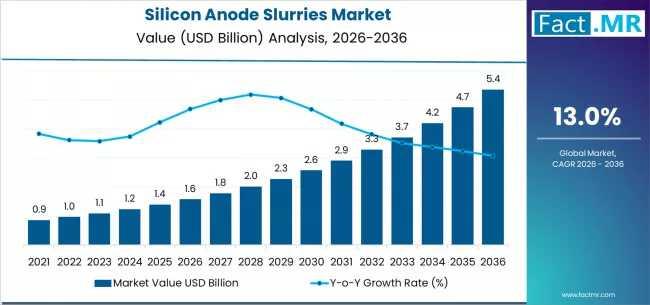
Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…

Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…
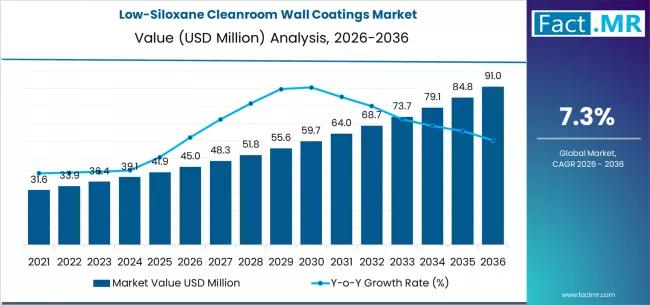
Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…

Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…
More Releases for PRP
Therapeutic PRP Tubes Market to Witness Growth at a 6.6% CAGR by 2031 | Regen La …
QYResearch A newly published report titled "Global Therapeutic PRP Tubes Market 2025 is carefully researched in the report while largely concentrating on top players and their business tactics, geographical expansion, market segments, competitive landscape, manufacturing, and pricing and cost structures. Each section of the research study is specially prepared to explore key aspects of the global Therapeutic PRP Tubes market. For instance, the market dynamics section digs deep into the…
Global PRP Centrifuges Market Research Report 2023-2029
Global Leading Market Research Publisher QYResearch announces the release of its lastest report "Global PRP Centrifuges Market Report, History and Forecast 2018-2029, Breakdown Data by Manufacturers, Key Regions, Types and Application". Based on historical analysis (2018-2022) and forecast calculations (2023-2029), this report provides a comprehensive analysis of the global PRP Centrifuges market, including market size, share, demand, industry development status, and forecasts for the next few years. Provides advanced statistics…
Latest Study on Platelet Rich Plasma (PRP) Market Future Growth Predictions and …
The Global Platelet Rich Plasma (PRP) Market 2019-2026 increase in the number of orthopedic surgeries and sports injuries and rise in the number of cosmetic surgeries are driving the growth of the market. However, high cost of devices & therapy will limit the market growth.
The Global Platelet Rich Plasma (PRP) Market 2019 report includes Platelet Rich Plasma (PRP) Market Revenue, market Share, industry volume, and Trends, Growth aspects. Growing prevalence…
Platelet Rich Plasma (PRP) Market is expected to grow at a CAGR of roughly 13.3% …
New Study Report On "Platelet Rich Plasma (PRP) Market is expected to grow at a CAGR of roughly 13.3% | Analysis By PRP Type (P-PRP, L-PRP, L-PRF), By Players (DePuy Synthes, Stryker...), By Surgery (Orthopedic, Cosmetic, General...) & Region, Size & Forecast To 2023"
The worldwide market for Platelet Rich Plasma (PRP) is expected to grow at a CAGR of roughly 13.3% over the next five years, will reach 400 million…
Platelet Rich Plasma Market By PRP Type (Pure-PRP, Leucocyte Rich PRP, Pure Plat …
Platelet rich plasma (PRP) is a portion of the plasma fraction of analogous blood, which contains platelet concentration more than standard concentration. Platelets contains bio proteins that helps in healing, tissue regeneration, and blood loss. Platelet rich plasma protein consists of three proteins, which acts as cell adhesive molecules that are fibrin, fibronectin, and vitronectin. The use of platelet rich plasma therapy is tremendously increased after 2009 due to its…
Platelet Rich Plasma (PRP) Market Forecast 2017-2021
Platelet rich plasma (PRP) market has witnessed appreciable growth due to a growing number of devices being FDA approved in recent years; however the therapeutics market is anticipated to have an uncertain future, even as non-approved therapeutic applications flourish. Although various platelet rich plasma isolation devices have received 510(k) clearance, platelet rich plasma has not been approved for direct injection or implant without prior mixing with the bone graft materials.…
