Press release
Portable Medical Devices Market is expected to reach USD 146.4 billion by 2030, growing at a CAGR of 11.3%, Diagnostic & Monitoring Devices segment holds 48.6% Market revenue. North America led 38.4% share of global market
The global portable medical devices market size was estimated at USD 63.0 billion in 2022 and is projected to reach USD 146.4 billion by 2030, growing at a CAGR of 11.3% from 2023 to 2030. An increase in chronic diseases due to changing lifestyles, a preference for home healthcare, and a rise in the geriatric population is anticipated to positively impact the market growth in the coming years.Download your exclusive sample report today: (corporate email gets priority access):https://www.datamintelligence.com/download-sample/portable-medical-devices-market?pratik
United States: Key Industry Developments
✅ November 2025: Cybersecurity compliance costs have risen significantly for manufacturers, with cyber-insurance premiums climbing 15% year-on-year in 2025, which is impacting the pricing of connected portable devices.
✅ October 2025: Market reports highlighted an increasing trend toward integrating AI and advanced data analytics directly into portable medical devices to improve diagnostic accuracy and support sustainable healthcare practices.
✅ September 2025: Regulatory alignment was noted in the U.S., where the FDA has cleared the first over-the-counter continuous glucose monitor and is actively embracing the "Home as a Health Care Hub" concept to validate remote monitoring technologies.
United States: Recent Product Launches
✅ June 2025: SoundHealth received FDA approval for its new product, the
Sonu Band, an AI-enabled wearable designed to treat moderate-to-severe nasal congestion in adolescents.
✅ April 2025: Dexcom received FDA clearance for its , an extended-life continuous glucose monitoring product for adults aged 18 and over.
✅ January 2025: Withings gained FDA clearance for its , a connected device that links ocular microvascular imaging with hypertension detection to help identify early signs of organ damage.
Japan: Key Industry Developments
✅ November 2025: The Japanese market is emphasizing the integration of AI and IoT technologies into portable devices to support an aging population, creating high demand for products offering early diagnosis and constant health monitoring.
✅ July 2025: Omron announced plans to build its first manufacturing plant in Chennai, India, a strategic move to boost the production of blood pressure monitors and ECG devices to meet rising home-care demand across the broader Asian market, which includes Japan.
Recent FDA Approval:-
→ November 17, 2025: Synova Life Sciences, Inc. received 510(k) clearance for their Synova Wave Adipose Processing System, a portable system used for processing adipose tissue. This device facilitates point-of-care procedures.
→ October 22, 2025: RMedical Co., Ltd. received 510(k) clearance for the
Dr. PRP-30 Automated blood cell separator. This portable device is designed for the safe and rapid preparation of autologous platelet-rich plasma at a patient's point of care.
→ October 3, 2025: B.T.I. Biotechnology Institute, S.L. received 510(k) clearance for the ENDORET® Kit. This kit is another portable system designed for point-of-care preparation of autologous platelet-rich plasma to be mixed with bone grafts.
→ September 10, 2025: Haemonetics Corporation received 510(k) clearance for SafeTrace Tx® Software 5.0.0, a web-based electronic information system for blood transfusion management. While software, it is a key component for managing data across potentially portable LIS systems in a blood banking environment.
→ August 28, 2025: StemC Biyoteknoloji Anonim Sirketi received 510(k) clearance for the Exocube Set, which is a portable platelet and plasma separator for bone graft handling at the patient's point of care.
→ August 26, 2025: Bio-Rad Laboratories, Inc. received 510(k) clearance for the GeeniusTM HIV 1/2 Supplemental Assay. This assay is a single-use, immunochromatographic test for confirmation of HIV antibodies using various portable samples including fingerstick whole blood.
Why Purchase the Report?
→ To visualize the global portable medical devices market segmentation based on device type, application, end user and region as well as understand key commercial assets and players.
→ Identify commercial opportunities by analyzing trends and co-development.
Excel data sheet with numerous data points of global portable medical devices market-level with all segments.
→ PDF report consists of a comprehensive analysis after exhaustive qualitative interviews and an in-depth study.
→ Product mapping available as Excel consisting of key products of all the major players.
→ The global portable medical devices market report would provide approximately 61 tables, 58 figures, and 185 Pages.
"Secure your 30% year-end discount - get this report before the offer expires."
:https://www.datamintelligence.com/buy-now-page?report=portable-medical-devices-market?pratik ((Purchase 2 or more Repots and get 50% Discount)
Market Segmenatation-
Segmentation by Product Type
→ Diagnostic & Monitoring Devices
This segment includes portable ECG machines, glucose monitors, blood pressure monitors, pulse oximeters, wearable health trackers, and portable imaging tools. These devices support continuous or remote monitoring, driven by rising chronic diseases and home-based care adoption.It is the largest product segment, holding around 48.6% of the market share in 2025.
→ Therapeutic Devices
This segment consists of portable ventilators, insulin pumps, TENS devices, infusion pumps, and respiratory therapy tools. Demand is supported by the shift toward outpatient treatment, elderly care needs, and chronic respiratory illness management.Therapeutic devices account for approximately 32.4% of the market in 2025.
→ Fitness & Wellness Devices
This includes smart wearables, activity trackers, portable body analyzers, and digital fitness tools aimed at preventive health and consumer wellness. Growing interest in health optimization and lifestyle tracking contributes to strong growth.Fitness & wellness devices hold about 19% of the market share in 2025.
Segmentation by Application
→ Home Healthcare
This segment covers portable devices used at home for chronic disease monitoring, elderly care, rehabilitation, and personal wellness tracking. Rising healthcare costs and the expansion of telehealth strengthen demand.It is the dominant application segment with an estimated 61.2% market share in 2025.
→ Hospitals & Clinics
Hospitals use portable medical devices for bedside monitoring, emergency response, point-of-care diagnostics, and mobility-based treatment support. Increased adoption of portable ultrasound, ventilators, and diagnostic tools drives this segment.This segment holds around 28.7% of the market in 2025.
→ Ambulatory Surgical Centers (ASCs) & Emergency Services
This includes devices used in ambulances, trauma units, and day-surgery centers to enable rapid diagnostics and mobility-based intervention. Growth is supported by the rising number of emergency cases, road accidents, and demand for fast diagnostic turnaround.This segment accounts for roughly 10.1% of the market in 2025.
Regional insights:-
1. North America - 38.4% Market Share
North America leads the global market due to strong adoption of home healthcare devices, a high prevalence of chronic diseases, and rapid integration of portable diagnostic and monitoring technologies. The presence of major manufacturers and growing telemedicine usage further strengthen regional dominance.
2. Europe - 27.1% Market Share
Europe holds the second-largest share, driven by aging populations, increased government support for digital health, and rising adoption of portable imaging and monitoring systems. Strong healthcare infrastructure and reimbursement policies also enhance market growth.
3. Asia-Pacific - 24.6% Market Share
Asia-Pacific is the fastest-growing region due to rapid expansion of healthcare facilities, rising consumer awareness, and growing demand for affordable portable devices in China, India, and Southeast Asia. The region benefits from increasing investment in homecare technologies and wearable health monitoring.
4. Latin America - 6.1% Market Share
Latin America shows rising adoption of portable diagnostic and therapeutic devices driven by growing urbanization, government health initiatives, and expanding private healthcare services. Market growth is supported by increasing chronic disease burden and demand for cost-effective solutions.
Get Customization in the report as per your requirements:https://www.datamintelligence.com/customize/portable-medical-devices-market?pratik
Competitive Landscape
→ The major global players in the market include Lepu Medical Technology Co Ltd., Medtronic Plc., Becton Dickinson and Co, Omron Corporation, Koninklijke Philips NV, Hologic Inc., GE HealthCare Technologies Inc., Abbott Laboratories, Siemens Healthineers AG, FUJIFILM Holdings Corporation among others.
Key Developments
→ Mindray launched its first wireless, handheld ultrasound system called TE Air. TE Air features a wire-free design and compact size designed to easily fit into a pocket. It also offers high-level disinfection tolerance and flexible charging for extreme mobility.
→ Exo Imaging secured $200M in Series C financing aimed at commercializing its handheld ultrasound device and point-of-care workflow platform called Exo Works. The new funding also gives the Redwood City, California-based company over $320 million in total investments.
Request for 2 Days FREE Trial Access:
https://www.datamintelligence.com/reports-subscription?pratik
✅ Competitive Landscape
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Consumer Behavior & Demand Analysis
✅ Import-Export Data Monitoring
✅ Live Market & Pricing Trends
Have a look at our Subscription Dashboard:
https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About DataM Intelligence
DataM Intelligence is a renowned provider of market research, delivering deep insightsthrough pricing analysis, market share breakdowns, and competitive intelligence. Thecompany specializes in strategic reports that guide businesses in high-growth sectors suchas nutraceuticals and AI-driven health innovations.
To find out more, visit https://www.datamintelligence.com/ or follow us on Twitter,LinkedIn and Facebook.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Portable Medical Devices Market is expected to reach USD 146.4 billion by 2030, growing at a CAGR of 11.3%, Diagnostic & Monitoring Devices segment holds 48.6% Market revenue. North America led 38.4% share of global market here
News-ID: 4300331 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

Renewable Diesel Market is set to reach US$ 48.33 billion by 2032, North America …
Renewable Diesel Market size reached US$ 24.67 billion in 2024 and is expected to reach US$ 48.33 billion by 2032, growing with a CAGR of 8.77% during the forecast period 2025-2032.
Renewable Diesel Market growth is driven by stricter emission regulations, rising decarbonization goals, increasing availability of waste-based feedstocks, superior fuel performance, and growing adoption across transportation, power generation, and industrial sectors worldwide.
Get a Free Sample PDF Of This Report (Get…

United States Water Enhancers Market to hit US$ 2.57 Billion by 2031| North Amer …
Leander, Texas and Tokyo, Japan - Jan.29.2026
As per DataM intelligence research report "Global Water Enhancers Market reached US$ 4.81 billion in 2022 and is expected to reach US$ 8.57 billion by 2031, growing with a CAGR of 7.5% during the forecast period 2024-2031."
Growth is supported by rising consumer preference for flavored and functional beverages. Liquid water enhancers dominate due to convenience and customization. Health-conscious consumption trends and demand for low-calorie…
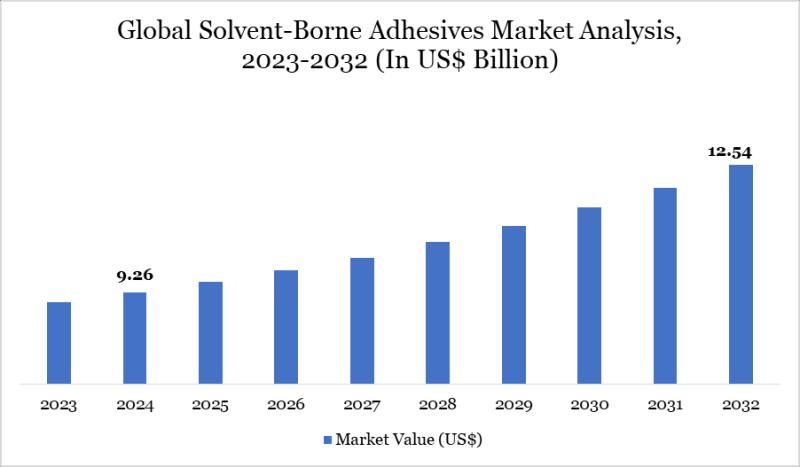
United States Solvent-Borne Adhesives Market to Reach US$ 3.76 Billion by 2032| …
Leander, Texas and Tokyo, Japan - Jan.29.2026
As per DataM intelligence research report "Solvent Borne Adhesives Market size reached US$9.26 billion in 2024 and is expected to reach US$12.54 billion by 2032, growing with a CAGR of 3.86% during the forecast period 2025-2032"
The market is driven by strong demand from packaging, automotive, and construction industries. High bonding strength and fast curing properties support adoption. Despite environmental regulations, demand remains strong in…

eVTOL Aircraft Market Set for Explosive Growth to USD 5,664.42 Billion by 2032, …
The Global eVTOL Aircraft Market reached USD 596.25 million in 2024 and is expected to reach USD 5,664.42 billion by 2032, growing with a CAGR of 32.50% during the forecast period 2025-2032.
Market growth is driven by rapid urbanization, surging demand for sustainable urban air mobility solutions, and advancements in electric propulsion technologies. Expanding investments from aerospace giants like Joby Aviation and Archer Aviation, supportive regulatory frameworks from FAA and EASA…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
