Press release
Graves Ophthalmopathy Treatment Market: Emerging Therapies & Key Insights, Key Market Updates 2025 | Immunovant, Inc., Viridian Therapeutics, Inc., Argenx, Tourmaline Bio, Inc., Sling Therapeutics
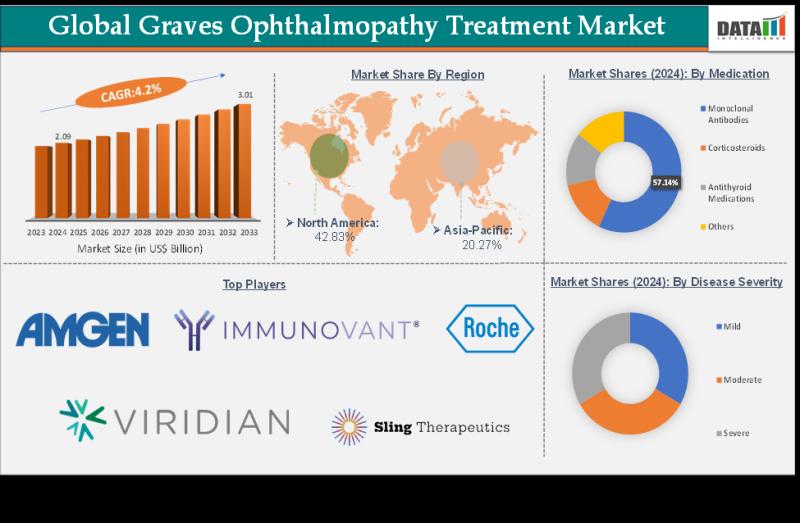
graves ophthalmopathy treatment market size reached US$ 2.09 Billion in 2024 and is expected to reach US$ 3.01 Billion by 2033, growing at a CAGR of 4.2% during the forecast period 2025-2033.DataM Intelligence has published a new research report on "Graves Ophthalmopathy Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/graves-ophthalmopathy-treatment-market?kb
Latest M&A Activity
Novartis agreed to acquire Tourmaline Bio for approximately USD 1.4 billion in Q4 2025. This deal strengthens Novartis' position in autoimmune and inflammatory diseases, supporting expansion into next-gen GO therapies.
Multiple pharmaceutical companies are investing in targeted immunotherapies and biologics for GO, including small molecule inhibitors and monoclonal antibodies focusing on immune and inflammatory pathways.
Key Players:
Amgen Inc., Immunovant, Inc., Viridian Therapeutics, Inc., Argenx, Tourmaline Bio, Inc., F. Hoffmann-La Roche Ltd and Sling Therapeutics
Key Development:
In January 2025, Sling Therapeutics, Inc. reported topline efficacy and safety results from the Phase 2b/3 LIDS trial of linsitinib in patients with active, moderate-to-severe thyroid eye disease (TED).
In May 2025, Viridian Therapeutics, Inc. announced that the U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy Designation to veligrotug, the company's lead anti-insulin-like growth factor-1 receptor (IGF-1R) candidate for TED treatment.
Historically, TED treatment options were limited to high-dose corticosteroids, radiation, or surgery, often yielding suboptimal outcomes and relapse rates of 30-40%. The 2020 approval of Tepezza (teprotumumab), the first targeted biologic therapy, spurred significant R&D investment in TED. However, Tepezza's IV-only administration, high cost ($14,900 per vial), and side effects such as hearing impairment highlighted the need for innovation. This has accelerated the development of next-generation therapies that are subcutaneous, oral, or utilize novel mechanisms of action.
Growth Forecast Projected:
The Global Graves Ophthalmopathy Market is anticipated to rise at a considerable rate during the forecast period, between 2025 and 2032. In 2024, the market is growing at a steady rate, and with the rising adoption of strategies by key players, the market is expected to rise over the projected horizon.
Research Process:
Both primary and secondary data sources have been used in the global Graves Ophthalmopathy Market research report. During the research process, a wide range of industry-affecting factors are examined, including governmental regulations, market conditions, competitive levels, historical data, market situation, technological advancements, upcoming developments, in related businesses, as well as market volatility, prospects, potential barriers, and challenges.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=graves-ophthalmopathy-treatment-market?kb
Key Segments:
By Medication: (Monoclonal Antibodies, Corticosteroids, Antithyroid Medications and Others)
By Disease Severity: (Mild, Moderate and Severe)
By End-User: (Hospitals, Specialty Clinics, Academic and Research Institutes and Others)
Recent Product Launches and Pipeline
Teprotumumab (Tepezza) remains the only FDA-approved treatment for GO. It is a fully human monoclonal antibody inhibiting the insulin-like growth factor-1 receptor (IGF-1R) and has demonstrated significant improvements in proptosis, eye inflammation, and diplopia in clinical trials.
Emerging candidates include Veligrotug (VRDN-001) by Viridian Therapeutics, a monoclonal antibody targeting IgG recycling via the neonatal Fc receptor (FcRn), showing strong clinical efficacy and receiving FDA Breakthrough Therapy Designation in May 2025; US launch planned for H2 2026.
Other developing therapies include Batoclimab (Immunovant Sciences) targeting FcRn, Efgartigimod PH20 SC (argenx), and novel JAK-STAT inhibitors targeting inflammatory signaling in GO.
There are ongoing Phase III trials for chronic and active TED patients, moving towards better-tolerated, earlier-intervention therapeutics.
Get Customization in the report as per your requirements: https://datamintelligence.com/customize/graves-ophthalmopathy-treatment-market?kb
Latest FDA Approvals and Regulatory Updates
No new FDA approvals beyond Teprotumumab occurred during 2025 yet, but multiple investigational therapies are in late-stage testing with anticipated submissions 2025-2026.
FDA has continued to update regulatory guidance emphasizing expedited pathways for autoimmune and rare disease treatments including GO
Have any Enquiry of This Report @ https://www.datamintelligence.com/enquiry/graves-ophthalmopathy-treatment-market?kb
2025 is a transformative year in Graves Ophthalmopathy treatment characterized by:
Strategic M&A deals like Novartis-Tourmaline Bio expanding biologic portfolios targeting TED.
Established efficacy of Teprotumumab as the first FDA-approved, IGF-1R inhibitory therapy demonstrating durable improvement in proptosis and orbital inflammation.
Promising investigational therapies like Veligrotug poised for launch with innovative FcRn-targeted mechanisms.
Emerging pipeline therapies aimed at improving safety, patient comfort, and addressing chronic stages of the disease.
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Graves Ophthalmopathy Treatment Market: Emerging Therapies & Key Insights, Key Market Updates 2025 | Immunovant, Inc., Viridian Therapeutics, Inc., Argenx, Tourmaline Bio, Inc., Sling Therapeutics here
News-ID: 4236207 • Views: …
More Releases from DataM Intelligence 4 Market Research LLP

AI in Oil and Gas Market Set for Explosive Growth to USD 15.0 billion by 2035, L …
Market Overview
The AI in Oil and Gas Market is estimated to be valued at USD 4.2 billion in 2025 and is projected to reach USD 15.0 billion by 2035, registering a compound annual growth rate (CAGR) of 14.1% over the forecast period.
The AI in oil and gas market is growing rapidly, driven by the industry's focus on operational efficiency, predictive maintenance, and cost optimization amid volatile energy prices. Artificial intelligence…
Targeted Protein Degradation Market to Reach USD 4.53 Billion by 2033 at 32.4% C …
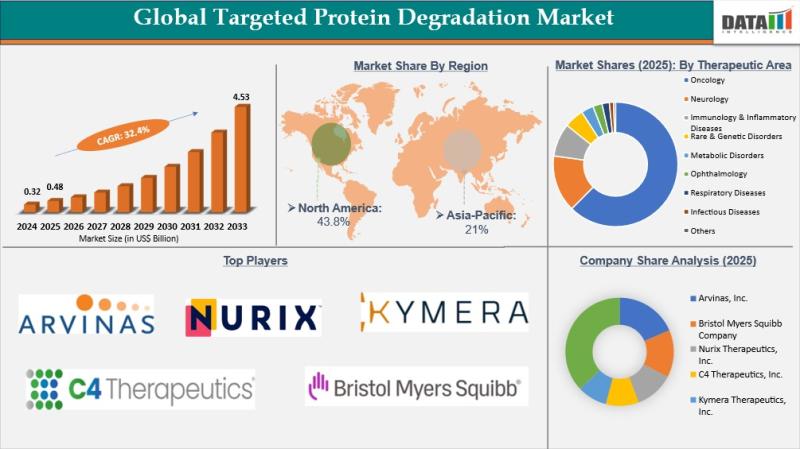
The Targeted Protein Degradation Market was valued at USD 0.32 billion in 2024, rising to USD 0.48 billion in 2025, and is projected to reach USD 4.53 billion by 2033, expanding at a CAGR of 32.4% during the forecast period from 2026 to 2033. This rapid growth is strongly driven by the substantial global burden of hormone receptor positive breast cancer and the clinical limitations of existing endocrine therapies. According…

Neobanking Market to Reach USD 364.18 Billion by 2033 at 35.12% CAGR | North Ame …
The Neobanking Market was valued at USD 36.22 billion in 2024 and is projected to reach USD 364.18 billion by 2033, expanding at a CAGR of 35.12% during the forecast period from 2025 to 2033. This exceptional growth reflects accelerating global adoption of digital-first banking solutions that offer streamlined, customer-centric financial services without traditional branch infrastructure. Neobanks are revolutionizing banking by delivering seamless mobile and web-based experiences with lower fees,…
Electronic Clinical Outcome Assessment Solutions Market Set for Explosive Growth …
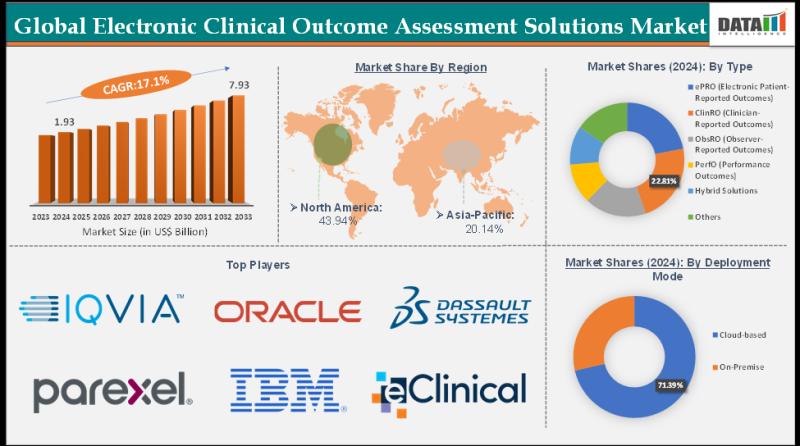
The Global Electronic Clinical Outcome Assessment Solutions Market size reached US$ 1.93 Billion in 2024 from US$ 1.67 Billion in 2023 and is expected to reach US$ 7.93 Billion by 2033, growing at a CAGR of 17.1% during the forecast period 2025-2033.
Market growth is driven by the surging demand for patient-centric clinical trials, regulatory mandates for electronic data capture (e.g., FDA's eCOA guidelines), and the need for real-time, accurate patient-reported…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…