Press release
Antibody Validation Market Analysis Highlights Future Scope - Persistence Market Research
✅ Antibody Validation Market: An In-Depth Analysis of Growth Trends and Market DynamicsThe antibody validation market is emerging as a pivotal component in the life sciences sector, driven by the need for reproducibility and accuracy in antibody-based research. With antibodies playing a critical role in diagnostics, therapeutics, and drug discovery, the validation process has become indispensable. Market validation ensures that antibodies used in various assays meet stringent performance standards. According to recent estimates, the antibody validation market is expected to witness a steady CAGR through 2032, spurred by rising investments in life science research and growing concerns about reproducibility crises in biomedical studies.
The reagents segment currently dominates the product category due to their widespread application and recurring use in labs, whereas North America leads geographically. This dominance is attributed to the region's high concentration of pharmaceutical companies, cutting-edge biotechnology firms, and expansive R&D infrastructure. Increasing awareness among researchers regarding the substandard quality of commercially available antibodies is one of the major drivers behind this trend. The COVID-19 pandemic, although initially disruptive, has also accelerated the need for high-quality antibodies, especially in vaccine development and diagnostics, thereby increasing the focus on validation.
✅Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/32058
✅ Key Highlights from the Report
➤ North America holds the largest market share due to intensive R&D activities in life sciences.
➤ Reagents are the leading product type owing to their frequent use and high demand in validation procedures.
➤ Growing awareness of reproducibility and antibody reliability is driving validation processes.
➤ Industry-led initiatives like IWGAV are creating standards and boosting global demand.
➤ COVID-19 has intensified scrutiny over antibody quality, creating a ripple effect in validation needs.
✅ Market Segmentation: Understanding the Scope and Application
The antibody validation market can be segmented by product type, validation method, and end-user. Among product types, reagents and consumables remain the most utilized due to their crucial role in validation workflows. These products are frequently consumed in various assays such as ELISA, Western Blotting, and immunohistochemistry, making them essential in repeated testing scenarios. Primary instruments, though critical, contribute a smaller share owing to their higher cost and lower rate of replacement.
In terms of validation methods, the market includes basic validation, genetic validation, tagged protein validation, and others. Among these, genetic validation is gaining popularity for its ability to provide more conclusive results regarding antibody specificity. Institutions and CROs are adopting multi-faceted validation strategies, combining genetic and orthogonal approaches to ensure rigorous standards. Furthermore, end-users include academic institutions, pharmaceutical companies, and contract research organizations (CROs), with pharmaceutical and biotech firms leading the demand due to the high stakes involved in drug development and regulatory approval.
✅ Regional Insights: Market Distribution and Growth Potential
North America continues to dominate the global antibody validation market, accounting for the highest revenue share in 2024. The region's advanced research infrastructure, strong regulatory oversight, and focus on precision medicine contribute to this growth. The U.S., in particular, leads with substantial investments from both government and private sectors into life science research. Furthermore, North America has several key players headquartered within its borders, driving market competition and innovation.
Meanwhile, Europe holds the second-largest share, propelled by increasing collaborations between academia and industry. Countries such as Germany, the UK, and France are at the forefront, hosting numerous biomedical research initiatives. South Asia and East Asia are poised for rapid expansion in the forecast period, thanks to growing investments in healthcare infrastructure, emerging biotech hubs, and increased governmental support. However, Middle East & Africa remains underdeveloped due to resource limitations and lower investment in biotechnology.
✅ Market Drivers: Fueling the Rise of Antibody Validation
The market is significantly driven by growing concerns over reproducibility in research. Increasing incidents of experimental errors due to poor-quality antibodies have prompted both academic and commercial labs to prioritize antibody validation. Furthermore, the proliferation of biologics and antibody-based drugs has necessitated rigorous validation protocols to meet regulatory standards. The rise of personalized medicine and diagnostic precision is also contributing to market growth, demanding high specificity and sensitivity in antibody reagents.
✅ Market Restraints: Challenges Hampering Market Acceleration
Despite promising growth, the antibody validation market faces notable restraints. A major hurdle is the lack of industry-wide standards for antibody validation. This leads to inconsistent practices and results across different research labs. Additionally, the high cost of validation is another deterrent for manufacturers and small-scale labs. Validation processes require additional resources, time, and sophisticated instrumentation, which increases the financial burden. Moreover, opaque supply chains and fragmented distribution networks limit quality control and traceability in the production and validation cycle.
✅Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/32058
✅ Market Opportunities: Unlocking Future Potential
While current challenges persist, the antibody validation market is ripe with opportunities. The establishment of global frameworks such as the International Working Group on Antibody Validation (IWGAV) is a major step toward standardization. Emerging markets in Asia-Pacific offer growth potential due to expanding R&D capabilities and increasing foreign investments. Advancements in artificial intelligence and machine learning also present opportunities for automating and optimizing validation processes. Additionally, partnerships between academia and biotech firms can pave the way for innovative validation tools and protocols, addressing the current bottlenecks in the market.
✅ Frequently Asked Questions (FAQs)
➤ How big is the antibody validation market in 2024?
➤ What is the projected growth rate of the antibody validation market through 2032?
➤ Who are the key players in the global antibody validation market?
➤ What is the market forecast for antibody validation by 2032?
➤ Which region is estimated to dominate the antibody validation industry through the forecast period?
✅ Company Insights: Key Players in the Market
✦ OriGene Technologies
✦ LifeSpan BioSciences
✦ Rockland Immunochemicals
✦ Badrilla Ltd.
✦ Bio-Rad Laboratories
✦ Abcam plc
✦ Thermo Fisher Scientific
✦ Sigma-Aldrich Corporation (Merck Group)
✦ Cell Signaling Technology, Inc.
✦ Santa Cruz Biotechnology, Inc.
✅ Recent Developments in the Market
■ In 2024, Thermo Fisher Scientific launched an AI-driven antibody validation platform aimed at streamlining specificity testing.
■ OriGene Technologies announced a strategic partnership with a European CRO to implement multistep validation processes across all new antibody products.
✅ Reasons to Buy the Report
Provides in-depth insights into the growing importance of antibody validation in life sciences.
Offers a clear segmentation of the market to help stakeholders identify key growth areas.
Includes regional analysis to guide geographic investment and expansion strategies.
Highlights emerging technologies and validation methods that are redefining the market.
Lists key players and recent innovations to assess the competitive landscape effectively.
The antibody validation market is poised for sustained growth as the need for quality, reproducibility, and regulatory compliance in life sciences research becomes more pronounced. With strong drivers like increasing research awareness, supportive frameworks, and technological innovation, the market is moving toward a more standardized and efficient validation ecosystem. Stakeholders in the biotech, pharmaceutical, and academic sectors will find compelling reasons to invest in and adopt advanced validation tools, ensuring that the antibodies powering today's discoveries are both reliable and effective.
Contact Us:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Antibody Validation Market Analysis Highlights Future Scope - Persistence Market Research here
News-ID: 4045728 • Views: …
More Releases from Persistence Market Research

Crab Meat Market Value to Reach US$1,691.6 Mn by 2033 Driven by Demand at 5.1% C …
The global crab meat market is poised for stable and sustained growth over the forecast period, driven by increasing consumer demand for premium seafood products, rising health consciousness, and expanding distribution networks. The market is projected to be valued at US$ 1,194.2 million in 2026 and is anticipated to reach approximately US$ 1,691.6 million by 2033, expanding at a compound annual growth rate (CAGR) of 5.1% from 2026 to 2033.…

PDC Drill Bits Market Size to Reach US$ 8.1 Billion by 2033 - Persistence Market …
The polycrystalline diamond compact PDC drill bits market plays a critical role in modern drilling operations across oil and gas mining and geothermal industries. PDC drill bits are engineered using synthetic diamond cutters bonded onto tungsten carbide substrates delivering exceptional hardness wear resistance and cutting efficiency. Compared to traditional roller cone drill bits PDC drill bits offer higher rate of penetration longer service life and reduced downtime making them a…
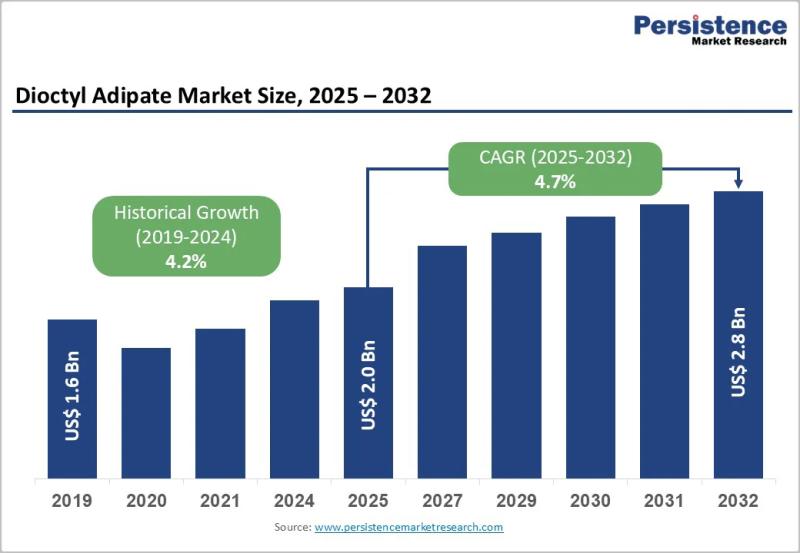
Dioctyl Adipate Market Valued at US$ 2.0 Billion in 2025 Projected to Reach US$ …
The dioctyl adipate market is gaining steady momentum as industries transition toward high performance and flexible plasticizer solutions that meet evolving regulatory and environmental standards. Dioctyl adipate, commonly referred to as DOA, is a widely used plasticizer known for its excellent low temperature flexibility, weather resistance, and compatibility with polyvinyl chloride and other polymers. It plays a critical role in the production of flexible PVC products, synthetic leather, food packaging…

Japan Disposable Cutlery Market to Reach US$103.9 Million by 2033 - Persistence …
The Japan disposable cutlery market represents a niche yet steadily expanding segment within the broader food service packaging industry. Disposable forks, spoons, knives, chopsticks, and hybrid utensil formats play an essential role in Japan's highly organized convenience driven food ecosystem. With one of the world's most advanced retail infrastructures and a strong culture of ready to eat meals, disposable cutlery has become an indispensable component of takeaway, food delivery, and…
More Releases for Validation
Global Email Validation Tools Market Size by Application, Type, and Geography: F …
USA, New Jersey- According to Market Research Intellect, the global Email Validation Tools market in the Internet, Communication and Technology category is projected to witness significant growth from 2025 to 2032. Market dynamics, technological advancements, and evolving consumer demand are expected to drive expansion during this period.
The email validation tools market is experiencing significant growth, driven by the increasing need for businesses to maintain clean and accurate email lists. With…
Bioprocess Validation Market Report Up to 2031
Visiongain has published a new report on Bioprocess Validation Market Report : Forecasts by Test Type (Extractables and Leachables, Integrity Testing, Microbiology Testing), Process Component (Filter Element, Bioreactors), End User (Biotechnology & Pharmaceutical Companies). PLUS Profiles of Leading Companies and Regional and Leading National Market Analysis. PLUS COVID-19 Recovery Scenarios.
Download Exclusive Sample of Report @ https://www.visiongain.com/report/bioprocess-validation-market-2021/#download_sampe_div
Validation is a method of building documentary evidence demonstrating a process, activity or procedure, carried…
AVB and TSN Validation for Networked Audio
TSN Systems presents comprehensive test suite for audio AVB/TSN validation in infotainment.
German measuring technology specialist TSN Systems presents an extensive AVB analysis suite, enabling precise testing complex of AVB / TSN communication in infotainment applications with its TSN Box 3.0 and TSN Tools products. This allows typical project teams in IVI development to master these technologies without having in-depth special knowledge in time-sensitive communication.
On the way to a service-oriented…
Cleanroom Monitoring & Validation in a Single System
Send Your Data to the Cloud for a Hassle-Free Audit
Whether you’re a Quality Engineer, Pharmaceutical Microbiologist or hospital staffer, proving cleanroom sterilization and decontamination is critical for both open and closed processes. Defining your monitoring points, sampling frequency, and documenting the results must all be part of your environmental monitoring plan. However, when performed manually, a high frequency of cleanroom measurements only lead to more contamination through human activity. To…
Data Loggers for HVAC Performance Validation
Fully-Automated Recording of Temperature & Humidity
Are you working in an HVAC application such as temperature monitoring in vents and ducts, humidity recording in greenhouses, or logging carbon dioxide levels in schools? Data loggers are an affordable way to automate environmental data collection for HVAC system validation. Compact and highly accurate, they’re ideal for monitoring indoor air quality and also for recording pressure, events, and more. CAS DataLoggers offers products from…
Belle Tire Passes AGRSS Validation
Belle Tire Auto Glass hosted a third party Automotive Glass Replacement Safety Standard (AGRSS) validation on Monday, January 18, 2010 and was found to be 100% compliant with the standard. This crowning achievement comes as the automotive glass division recently celebrated its second anniversary.
In announcing the validation result, John Cox, of Belle Tire Auto Glass stated, “Proving compliance with the safety standard through a third party validation firm is the…
