Press release
ECA Foundation announces Independent Authority Board (IAB) Members
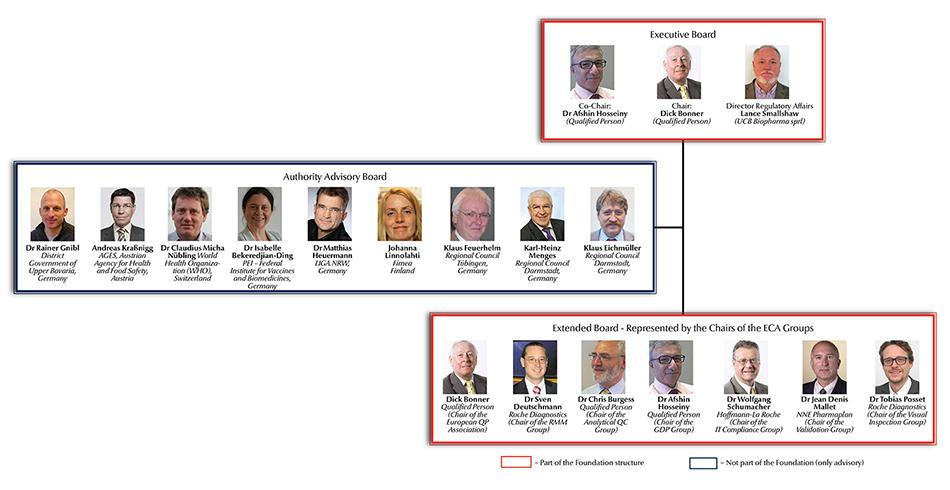
The ECA Foundation Structure with Executive Board, Extended Board and Independent Authority Board (IAB)
It is one of the ECA Foundation’s major objectives to discuss GMP developments with the stakeholders in industry, academia and authorities. For authority representatives any potential conflict of interest must be avoided. Therefore the ECA invited members of authorities to join the newly set up Independent Authority Board. This Board is not involved in any of the ECA activities, and its members are not part of the Foundation’s legal structure. This setup therefore now allows individual authority representatives to personally decide to get involved in discussions on a case by case basis. It also enables them to remain independent with regard to any ECA publication, comment or position paper – e.g. when the ECA is commenting on new regulations or when the ECA is publishing Best Practice Papers.
The newly set up Independent Authority Board comprises the following members:
• Dr Rainer Gnibl, Government of Upper Bavaria, Germany
• Andreas Kraßnigg, AGES (Austrian Agency for Health and Food Safety), Austria
• Dr Claudius Micha Nübling, World Health Organisation (WHO), Geneva
• Dr Isabelle Bekeredjian-Ding, Paul-Ehrlich-Institut (German Federal Institute for Vaccines and Biomedicines)
• Dr Matthias Heuermann, LIGA.NRW, Germany
• Johanna Linnolahti, Fimea Finland
• Klaus Feuerhelm, Regional Council Tübingen, Germany
• Karl-Heinz Menges, Regional Council Darmstadt, Germany
• Klaus Eichmüller, Regional Council Darmstadt, Germany
New structure paves way for future activities
Since its beginning in the year 1999 the ECA Foundation has developed like no other European organisation in the field of GMP compliance and pharmaceutical Quality Assurance. In mid 2015 the ECA Foundation therefore established the Executive Team with the Chairman, the Vice-Chairman and the Director Regulatory Affairs, the Extended Board with the various ECA Interest and Working Groups’ Chairmen and the aforementioned Independent Authority Board (IAB) as a new Advisory Committee.
With the Extended Board the Foundation intended to recognise and strengthen the Groups’ increasing importance and allow them to be directly involved in defining and planning ECA activities. Today the seven Interest and Working Groups build the broad basis for these activities:
The European Qualified Person Association (Interest Group) – 2400 Members
The Pharmaceutical Microbiology Group (Working Group) – 450 Members
The Analytical Quality Control Group (Working Group) – 140 Members
The Validation Group (Interest Group) – 190 Members
The Good Distribution Practices (GDP) Group (Interest Group) – 1730 Members
The Visual Inspection Group (Interest Group) – 1100 Members
The IT Compliance Group (Interest Group) – 240 Members
These groups’ goal is to actively drive various activities in specific areas – like organising trainings, conducting surveys to provide feedback to guideline drafts, writing Good Practice Guides or white papers.
In addition the ECA Foundation has established Europe’s largest Academy for GMP professionals – with annually more than 100 GMP events organised in 10 EU countries. Also, only in 2015 the Academy organised and conducted more than 50 in-house training courses for companies and European Authorities. And the trend is showing a further increase. Today the advanced training provider counts close to 4.000 members, and the number is still increasing.
Please find the ECA Foundation Executive and Extended Board structure below.
About the ECA Foundation
Founded as an independent not for profit organisation in 1999, the ECA Foundation is Europe’s leading association for pharmaceutical Quality Assurance and GMP compliance. Its goal is “the exchange of information between representatives of the industry, regulatory authorities and universities in the field of pharmaceutical quality assurance, especially with regard to the area of Good Manufacturing Practice (GMP).” For that purpose the organisation has set up several Interest Groups and Working Groups as well as an Academy for advanced education. Individuals can become member of the ECA Groups and in the Academy. In total more than 7.000 professionals have joined the ECA organisation. Its largest group, the European Qualified Person Association, alone counts close to 2.400 QPs. The ECA Foundation Groups developed a range of tools like several Good Practice Guides and the GMP Guideline Manager CD-ROM. For further information on the ECA Foundation please visit www.eca-foundation.org.
Wolfgang Heimes
Administration Manager
ECA Foundation
P.O. Box 10 21 68
69011 Heidelberg, Germany
www.eca-foundation.org
www.gmp-compliance.org
heimes@gmp-compliance.org
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release ECA Foundation announces Independent Authority Board (IAB) Members here
News-ID: 351020 • Views: …
More Releases from ECA Foundation

Paul-Ehrlich-Institute and ECA Foundation jointly organise 2-day Workshop – Go …
Vaccine development and deployment are crucial to control the COVID-19 pandemic. Platform technologies have enabled rapid development of vaccines with demonstrated efficacy against COVID-19. However, large-scale manufacturing is both a prerequisite and a challenge in meeting the global demand. This implies upscaling of manufacturing, meeting Good Manufacturing Practice (GMP) requirements, and switching of manufacturing in existing facilities.
The workshop organised by the Paul-Ehrlich-Institut and the ECA Foundation will address these…

ECA Foundation added to EMA List of Stakeholder Organisations
The ECA Foundation is now listed in the European Medicines Agency's (EMA) list of eligible industry stakeholder organisations (www.ema.europa.eu/en/documents/other/list-eligible-industry-stakeholder-organisations_en.pdf). Pre-requisite for this entry is the listing in the EU's Transparency Register (http://ec.europa.eu/transparencyregister/public/consultation/searchControllerPager.do?declaration=ECA%20Foundation&search=search&locale=en#en) - where the ECA has been registered since January 2019.
The ECA Foundation is the leading European organisation with regard to pharmaceutical Quality Assurance and GMP compliance.
The ECA Foundation is a not for profit organisation. The ECA is headed…
European GDP Association nominates new Advisory Board Member
So far the Board of the European GDP Association comprised four members from the industry side, supported by a representative from the Finnish Medicines Agency FIMEA. Now a new Board Member has been nominated on the industry side.
Dr Laura Ribeiro, who is a Responsible Person at ID Logistics (formerly Logiters) in Portugal, has recently accepted her nomination as the fifth member of the GDP Association's Board. Prior to her…
More Releases for Board
Fine Pitch Board to Board Connector Market
Fine Pitch Board to Board Connector Market Overview
Fine Pitch Board to Board Connector are used in a stackable form for precise interconnection between printed circuit boards (PCBs). They can accommodate various types of PCB orientations, relative distances, and space constraints for design flexibility. Additionally, a variety of materials, platings, spacings and heights are available to meet electrical and mechanical specifications. Based on different interconnection purposes, small board-to-board connectors also support…
CMR Institute's Board of Directors Welcomes New Board Member
Roanoke, VA - November 3, 2023 CMR Institute's Board of Directors recently voted in a new board member, Dr. Anthony N. Akosa, MD, MBA, System Medical Director of Franciscan Alliance Care Management at Franciscan Alliance, Inc.
"On behalf of the Board, I am thrilled to welcome Anthony to the CMR Institute Board of Directors. His experience as an executive healthcare leader and provider will strengthen our ability to lead and support…
Global Backer Board Market, Global Backer Board Industry, Covid-19 Impact Global …
The Backer Board market is expected to grow from USD X.X million in 2020 to USD X.X million by 2026, at a CAGR of X.X% during the forecast period. The Global Backer Board Market report is a comprehensive research that focuses on the overall consumption structure, development trends, sales models and sales of top countries in the global Backer Board market. The report focuses on well-known providers in the global…
Global Board-to-board (BTB) Connectors Market Research Report
This report studies the global Board-to-board (BTB) Connectors market status and forecast, categorizes the global Board-to-board (BTB) Connectors market size (value & volume) by manufacturers, type, application, and region. This report focuses on the top manufacturers in United States, Europe, China, Japan, South Korea and Taiwan and other regions
Get sample copy of the report:
https://www.marketdensity.com/contact?ref=Sample&reportid=77869
Table of Contents:
Table of Contents
Global Board-to-board (BTB) Connectors Market Research Report 2018
1 Board-to-board (BTB) Connectors Market Overview
…
Automotive Connectors Market 2021 : Wire to Wire, Wire to Board, Board to Board
RnRMarketResearch Add New “Automotive Connectors Market 2021 : Wire to Wire, Wire to Board, Board to Board” Industry Research Report to It’s a Database.
Download sample copy of This Report @ https://goo.gl/dzvCeB
The automotive connectors market was estimated to be USD 4.38 billion in 2016, and is projected to reach USD 6.28 billion by 2021.The market, in terms of value, is projected to grow at a CAGR of 7.46%…
Global Magnesium Fireproof Board Market: Framecad, Futai Decorative Board, Huach …
Global Magnesium Fireproof Board Market report 2017 is an in-depth research on the current situation of the Magnesium Fireproof Board industry.
The Scope of the Magnesium Fireproof Board research report:
The Global Magnesium Fireproof Board Market primarily includes a basic overview of the Magnesium Fireproof Board industry. It also includes Magnesium Fireproof Board definitions, classifications and applications. It segments the market by applications, types, regions, competitive players and also analyzes Magnesium Fireproof…