Press release
ECA Foundation added to EMA List of Stakeholder Organisations
The ECA Foundation is now listed in the European Medicines Agency's (EMA) list of eligible industry stakeholder organisations (www.ema.europa.eu/en/documents/other/list-eligible-industry-stakeholder-organisations_en.pdf). Pre-requisite for this entry is the listing in the EU's Transparency Register (http://ec.europa.eu/transparencyregister/public/consultation/searchControllerPager.do?declaration=ECA%20Foundation&search=search&locale=en#en) - where the ECA has been registered since January 2019.The ECA Foundation is the leading European organisation with regard to pharmaceutical Quality Assurance and GMP compliance.
The ECA Foundation is a not for profit organisation. The ECA is headed by a Foundation Board and a Trustee. The legal status is a Sheltered Foundation which is controlled by a lawyer who acts as the Trustee of the Foundation. The Trustee is responsible for the supervision of the statutes (charter) of the organisation. The official representative of the organisation is the Chair (Dr Afshin Hosseiny). Please see the ECA Foundation website (www.eca-foundation.org) for more detailed information. The ECA Foundation is the legal entity and heads a number of Interest Groups. The largest Interest Group is the European QP Association which represents more than 3.000 Qualified Persons in Europe.
With the listing the EMA confirmed that the ECA fulfills the criteria and will now be contacted for involvement on all four levels defined by the agency:
1. Inform (to enable feedback e.g. news items, Q&As, information Day);
2. Consult (via written consultation e.g. guidelines development, public consultations on deliverables);
3. Consult & Involve (based on direct interactions e.g. focus groups) and,
4. Co-operate (jointly engaging towards a common technical goal e.g. technical expert groups).
The first 2 levels of stakeholder involvement are open to all external parties/stakeholders and do not require specific eligibility criteria to be applied. On these two levels the ECA and its European QP Association have already been registered for several years and have thus been receiving information and notice of written consultations in selective areas.
The eligibility criteria for levels 3 and 4 apply where an organisation seeks direct involvement in EMA activities, i.e. where the industry stakeholder is consulted and involved directly or co-operating with the Agency in specific areas - and requires to submit written declaration/confirmation that the eligibility criteria defined are fulfilled.
ECA Foundation
P.O. Box 10 21 68
69011 Heidelberg
Germany
www.eca-foundation.eu
www.gmp-compliance.org
Contact:
Wolfgang Heimes
Phone +49 (0) 6221 39 20 906
Fax +49 (0) 6221 39 20 907
heimes@gmp-compliance.org
The ECA Foundation is the leading European organisation with regard to pharmaceutical Quality Assurance and GMP compliance.
The ECA Foundation is a not for profit organisation. The ECA is headed by a Foundation Board and a Trustee. The legal status is a Sheltered Foundation which is controlled by a lawyer who acts as the Trustee of the Foundation. The Trustee is responsible for the supervision of the statutes (charter) of the organisation. The official representative of the organisation is the Chair (Dr Afshin Hosseiny).
It is comprised of an educational organisation (ECA Academy) and various non-profit interest and working groups. The modern structure of the ECA Foundation allows to develop and include the activities of these groups and to have more flexibility to build new groups that support the needs of our ECA Academy’s members. Close to 4,000 members in the Foundation’s educational organisation ECA Academy from all over Europe and abroad represent more than 60 countries.
www.eca-foundation.org
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release ECA Foundation added to EMA List of Stakeholder Organisations here
News-ID: 1819735 • Views: …
More Releases from ECA Foundation

Paul-Ehrlich-Institute and ECA Foundation jointly organise 2-day Workshop – Go …
Vaccine development and deployment are crucial to control the COVID-19 pandemic. Platform technologies have enabled rapid development of vaccines with demonstrated efficacy against COVID-19. However, large-scale manufacturing is both a prerequisite and a challenge in meeting the global demand. This implies upscaling of manufacturing, meeting Good Manufacturing Practice (GMP) requirements, and switching of manufacturing in existing facilities.
The workshop organised by the Paul-Ehrlich-Institut and the ECA Foundation will address these…
European GDP Association nominates new Advisory Board Member
So far the Board of the European GDP Association comprised four members from the industry side, supported by a representative from the Finnish Medicines Agency FIMEA. Now a new Board Member has been nominated on the industry side.
Dr Laura Ribeiro, who is a Responsible Person at ID Logistics (formerly Logiters) in Portugal, has recently accepted her nomination as the fifth member of the GDP Association's Board. Prior to her…
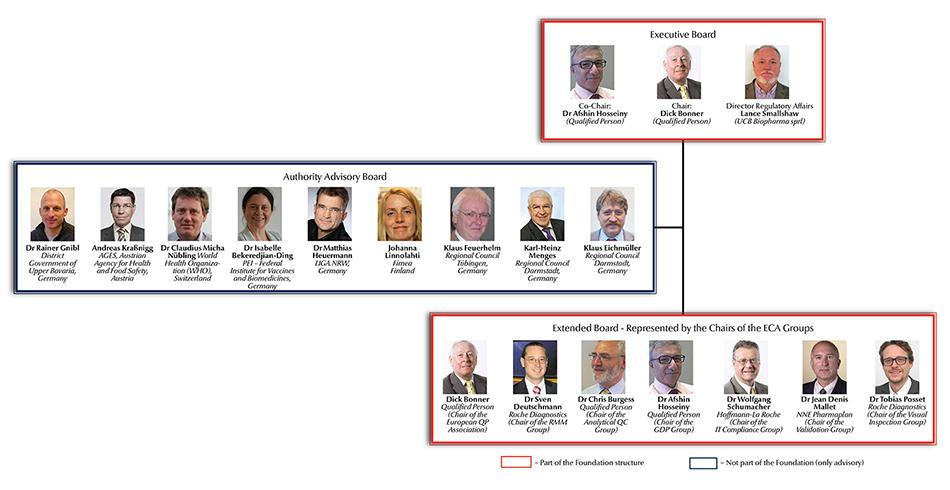
ECA Foundation announces Independent Authority Board (IAB) Members
Since June 2015 the ECA Foundation, the leading European organisation in the pharmaceutical quality assurance and GMP compliance environment, has been managed by an Executive Board and an Extended Board. In addition, the ECA set up an Advisory Committee encompassing authority representatives. Now the Foundation announced the members of the Independent Authority Board (IAB).
It is one of the ECA Foundation’s major objectives to discuss GMP developments with the stakeholders…
More Releases for Europe
2019 Strategy Consulting Market Analysis | McKinsey, The Boston Consulting Group …
Strategy Consulting Market reports also offer important insights which help the industry experts, product managers, CEOs, and business executives to draft their policies on various parameters including expansion, acquisition, and new product launch as well as analyzing and understanding the market trends
Need for strategic planning in highly competitive environment and to develop business capabilities to meet & exceed the emerging requirements are the major drivers which help in surging…
Strategy Consulting Market 2025 | Analysis By Top Key Players: Booz & Co. , Rola …
Global Strategy Consulting Market 2019-2025, has been prepared based on an in-depth market analysis with inputs from industry experts. This report covers the market landscape and its growth prospects over the coming years. The report also includes a discussion of the key vendors operating in this market.
The key players covered in this study
McKinsey , The Boston Consulting Group , Bain & Company , Booz & Co. , Roland Berger Europe…
Digital Strategy Consulting Market is Thriving Worldwide with Deloitte, McKinsey …
A Digital Strategy is a form of strategic management and a business answer or response to a digital question, often best addressed as part of an overall business strategy. A digital strategy is often characterized by the application of new technologies to existing business activity and focus on the enablement of new digital capabilities to their business.
A new report as a Digital Strategy Consulting market that includes a comprehensive analysis…
Strategy Consulting Market 2019: By McKinsey, The Boston Consulting Group, Bain …
This report studies the global Strategy Consulting market, analyzes and researches the Strategy Consulting development status and forecast in United States, EU, Japan, China, India and Southeast Asia. This report focuses on the top players in global market, like
• McKinsey
• The Boston Consulting Group
• Bain & Company
• Booz & Co.
• Roland Berger Europe
• Oliver Wyman Europe
• A.T. Kearney Europe
• Deloitte
• Accenture Europe
Get Sample Report@ https://www.reporthive.com/enquiry.php?id=1247388&req_type=smpl&utm_source=AB
Market segment by Type, the product can be split into
• Operations Consultants
• Business Strategy Consultants
• Investment Consultants
• Sales and…
Strategy Consulting Market Analysis 2018: McKinsey, The Boston Consulting Group, …
Orbis Research Present’s “Global Strategy Consulting Market” magnify the decision making potentiality and helps to create an effective counter strategies to gain competitive advantage.
The global Strategy Consulting status, future forecast, growth opportunity, key market and key players. The study objectives are to present the Strategy Consulting development in United States, Europe and China.
In 2017, the global Strategy Consulting market size was million US$ and it is expected to reach million…
Influenza Vaccination Market Global Forecast 2018-25 Estimated with Top Key Play …
UpMarketResearch published an exclusive report on “Influenza Vaccination market” delivering key insights and providing a competitive advantage to clients through a detailed report. The report contains 115 pages which highly exhibits on current market analysis scenario, upcoming as well as future opportunities, revenue growth, pricing and profitability. This report focuses on the Influenza Vaccination market, especially in North America, Europe and Asia-Pacific, South America, Middle East and Africa. This…
