Press release
Sysmex Europe and GLP Systems to attend Euromedlab
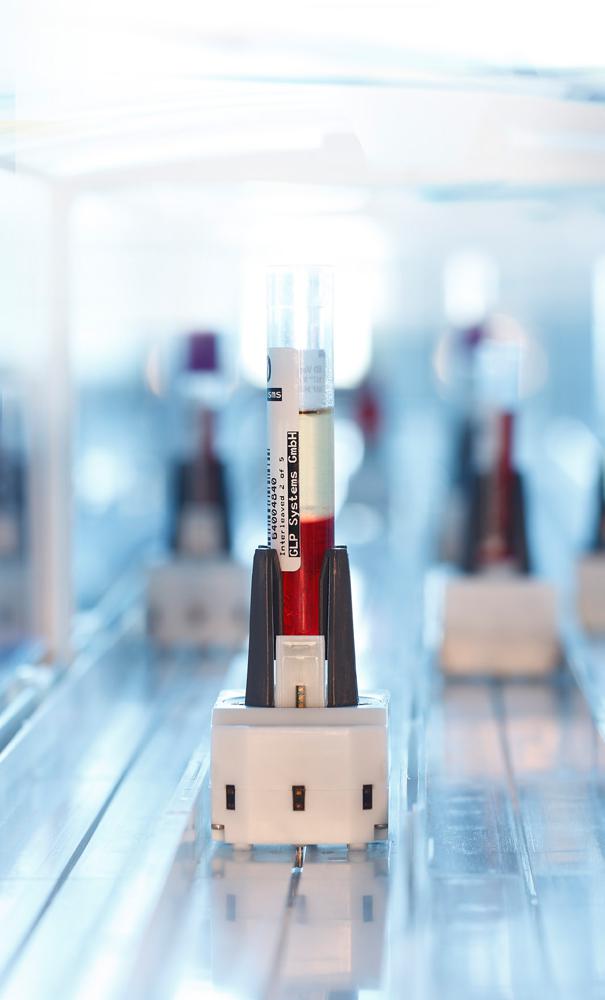
Norderstedt, Germany, 21 May 2013 – Healthcare solution specialist Sysmex Europe and lab automation experts GLP Systems recently announced their long-term cooperation in the field of laboratory automation. Now both companies are attending Euromedlab in Milan from 19-23 May – and are looking forward to answering any queries. With a brand new transport concept, modular solutions and real freedom from manufacturers, they are confident they are introducing a game changer in the lab. Lab owners will soon be able to create laboratory automation systems that meet their true needs.GLP Systems and Sysmex are delighted to be attending the Euromedlab fair and conference in Milan together - one of Europe’s leading platforms for clinical chemistry and laboratory medicine. With a real-life demonstration of the new track system, GLP Systems is looking forward to providing details about the new concept. One of the first solutions will be GLP’s superb island storage solution – available track-linked or as an island solution. It is fully automated and capable of storing up to 10,000 samples. The track is expected to steal the show, however, with its intelligent cars that transport individual samples dynamically to and from the various analysers and other devices.
One of the main changes is that lab owners can now choose a system that delivers complete independence from analyser manufacturers. This freedom of choice leads to full cost manageability. The system is also significantly faster than current solutions. Manageability and independence are further underlined by the fact that the system is entirely modular. It can be expanded as needed - from storage units only to total lab automation. This can cover all chemistries and sample volumes. Investments are secure for many years to come.
Both Sysmex and GLP Systems are passionate about using knowledge and experience to provide tangible, reliable solutions – for both existing and projected issues. The success in their approach lies in reducing complexity to a minimum, eliminating the traditionally weak links in the automation process and so creating new possibilities.
Despite the close operational cooperation, both companies continue to operate as independent entities.
About Sysmex
Sysmex is a global group, with more than 40 overseas subsidiaries conducting business in more than 170 countries. It develops, produces and sells instruments, reagents and software used in in-vitro diagnostics in hospitals and testing centres. In the global market, Sysmex ranks in the top 10 in diagnostics. In haematology, it holds the top share of the global market.
Overseas business development has been a focus for Sysmex since it was founded. It operates R&D, production, sales and customer support facilities around the world to ensure products and services are delivered that meet the needs of the customer in each region. Principal clinical areas of activity are: haematology, haemostasis, immunochemistry, urinalysis, clinical chemistry and life science. For more information please visit http://www.sysmex-europe.com
About GLP Systems
Located in Hamburg, Germany, GLP Systems is driven by the desire to eliminate the traditional limitations in lab automation. It is doing so by applying three basic principles: freedom, excellence and simplicity. The resulting solutions deliver superb levels of performance, choice and total manageability. The company draws on the experience, know-how and strength of its founders and shareholders, Dr. André von Froreich, Dr. Robert Hecht and Sonic Healthcare Germany.
www.glp-systems.com
Sysmex Europe GmbH
Bornbarch 1
22848 Norderstedt
luehmann.ines@sysmex-europe.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Sysmex Europe and GLP Systems to attend Euromedlab here
News-ID: 261346 • Views: …
More Releases from Sysmex Europe GmbH
Sysmex Medical Scientific Department Presentations at Euromedlab 2013
Norderstedt, Germany, 28 May 2013 - The Sysmex Europe Medical Scientific Department gave four exciting presentations at Euromedlab in Milan last week. They were attended by over 500 people across two sessions. The presentations addressed the latest findings in laboratory diagnostics developed in cooperation with Sysmex’s R&D and Clinical Management Concepts departments in Kobe, Japan. Those attending included prime decision-makers from laboratories across Europe and the world looking to improve…
More Releases for GLP
GLP Diet Review: Tailored Nutrition and Support for GLP-1 Users
Image: https://www.globalnewslines.com/uploads/2026/01/1768225059.jpg
GLP Diet Review - A Personalized GLP-1 Diet
The GLP Diet [https://glp.diet/?utm_source=abnewswire&utm_medium=organic&utm_campaign=bio] is a personalized wellness program designed to help users maximize the benefits of their GLP-1 weight loss medications. It offers tailored meal plans, balanced recipes, guided workouts, lifestyle challenges, and practical tools for tracking progress. Built around each user's unique needs, it turns complex nutrition advice into clear daily actions that support weight loss, better health, and long-term…
Alpharetta Medical Clinic Earns Top Recognition for Excellence in Peptide, GLP-1 …
Dr. Weight Loss of Atlanta in Alpharetta is proud to announce its recognition as the top-rated provider of medical weight loss and peptide therapy services in the Alpharetta area. This distinction reflects the clinic's sustained commitment to clinical excellence, patient-centered care, and successful treatment outcomes in the field of metabolic health.
Alpharetta, GA - December 16, 2025 - Dr. Weight Loss of Atlanta [https://drweightlossofatlanta.com/peptide-therapy-alpharetta] in Alpharetta is proud to announce its…
Medical Clinic Recognized as Top Peptide, GLP-1, & GLP-3 Therapies in Sandy Spri …
Dr. Weight Loss of Atlanta announced today that its Sandy Springs location has earned recognition as a leading medical weight loss and peptide therapy clinic in Sandy Springs, reflecting exceptional patient satisfaction, consistently strong clinical outcomes, and a reputation for delivering personalized, expert-guided care.
Sandy Springs, GA - December 16, 2025 - Dr. Weight Loss of Atlanta [https://drweightlossofatlanta.com/peptides-sandy-springs] announced today that its Sandy Springs location has earned recognition as a leading…
My Start GLP 1 Reviews: The GLP 1 That is Changing the Way America loss Weight
In the modern era of health innovation, few breakthroughs have generated as much excitement as My Start GLP 1. In 2025, it has rapidly become one of the most talked-about doctor-supervised weight loss programs in the United States. With thousands of verified patient success stories, My Start GLP 1 has positioned itself as more than a trend-it is a science-backed revolution changing how we understand metabolism, hunger, and sustainable fat…
GLP-1 Analogues Latest Market Analysis Report 2025
Global Info Research announces the release of the report "Global GLP-1 Analogues Market 2025 by Manufacturers, Regions, Type and Application, Forecast to 2031". This report provides a detailed overview of the GLP-1 Analogues market scenario, including a thorough analysis of the GLP-1 Analogues market size, sales quantity, average price, revenue, gross margin and market share.The GLP-1 Analogues report provides an in-depth analysis of the competitive landscape, manufacturer's profiles, regional and…
GLP-1 Market Taming Type 2 Diabetes and Obesity: The Therapeutic Promise of the …
GLP-1 Market Worth $95.4 Mn by 2031 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global GLP-1 Market Size, Share & Trends Analysis Report By Type of Molecule (Biologics and Small Molecules), Active Compound Used (Dulaglutide, Liraglutide, Orforglipron, Retatrutide, Semaglutide, Survodutide, Tirzepatide and Other Active Compounds),
Type of GLP-1 Agonist Drugs (Long-acting GLP-1 Agonist and Short-acting GLP-1 Agonist), Type…