Press release
ADC Has Curative Effect On Cell Surface Protein In Neuroblastoma
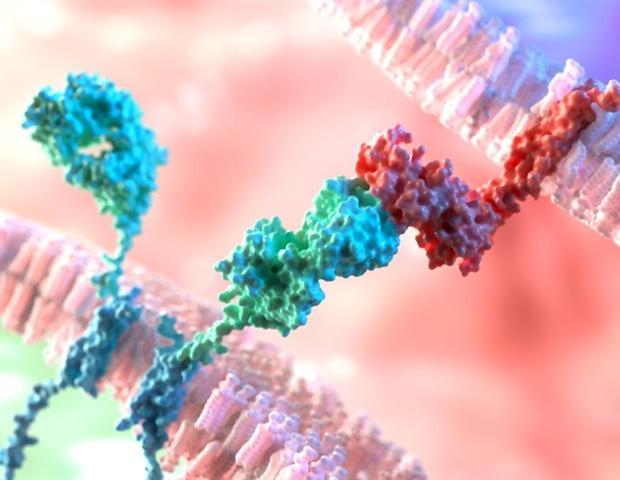
Physician-scientists in the Cancer Center at Children's Hospital of Philadelphia (CHOP) have developed a preclinical, potent therapy attached to an antibody that targets a surface protein expressed in most childhood neuroblastomas, effectively killing cancer cells.The researchers published their findings today in Science Translational Medicine.
In 2008, researchers discovered mutations in the anaplastic lymphoma kinase (ALK) gene as the major cause of the inherited form of neuroblastoma and showed that these same mutations are present in about 14 percent of neuroblastoma tumors from patients with the most aggressive form of this disease. This established ALK as a druggable target in neuroblastoma and provided the rationale for the clinical development of ALK inhibition therapy. It was found that native ALK (in the absence of a mutation) is present on most neuroblastoma tumors, providing us with an exciting opportunity to target ALK in the majority of patients.
Neuroblastoma, a pediatric cancer of the developing peripheral nervous system that usually occurs as a solid tumor in a child's chest or abdomen, is the most common cancer in infants, and accounts for more than 10 percent of all childhood cancer deaths. Children with the high-risk form of the disease continue to have a poor prognosis, despite intensive drug therapy.
Mossé, a pediatric oncologist and researcher at CHOP along with other scientists in her lab, demonstrated that the ALK protein appears on the surface of most neuroblastoma cells and is not detectable on normal cells, indicating that ALK is a useful target for immunotherapy. Researchers worked with pharmaceutical colleagues to weaponize an antibody-drug conjugate (ADC), one of a rapidly growing class of anticancer agents. That ADC, called CDX-0125-TEI, combines a specific monoclonal antibody engineered to recognize ALK with a potent chemotherapy drug—an alkylating agent called thienoindole (TEI).
In cell cultures and animal models of neuroblastoma, the ADC-ALK approach killed neuroblastoma cells, with no discernible toxicity to healthy cells. "The goal of this approach is to harness the presence of ALK to precisely deliver a poison only to the cancer cell, without harming the healthy surrounding cells," said Mossé.
"This study is proof of concept that the ALK protein is a good immunotherapy target and, as we optimize this approach for the clinic, has the potential to be useful for the majority of aggressive neuroblastomas and to minimize the harsh consequences of therapy," said Mossé.
She added, "In addition, our research could have great relevance for other cutting-edge immunotherapeutic strategies, such as CAR T-cell therapy, that harness the immune system to kill cancer, and is currently showing tremendous efficacy in children with leukemia. Our new data support clinical development of an immunotherapy drug not just for neuroblastoma, but for other hard-to-cure childhood cancers expressing the ALK gene, including rhabdomyosarcomas."
Biopharma PEG provides GMP standard PEG derivatives and bulk orders via custom synthesis, offering the opportunity to match customers’ special quality requirements. Biochempeg is dedicated to providing a chemical synthesis and high-quality ADC linkers. ADC linkers with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis.
Biopharma PEG Scientific Inc.
Address: 108 Water Street, Room 4D, Watertown, MA 02472, USA
TEL: 1-857-366-6766
Fax: 617-206-9595
Email: sales@biochempeg.com
Biopharma PEG is a biotechnology-oriented company in Watertown, Massachusetts. We are dedicated to manufacturing and supplying high purity polyethylene glycol (PEG) derivatives and reagents, monodisperse PEG, Click Chemistry reagents, PEGylation services and custom PEG derivative synthesis to clients worldwide. These PEG reagents have been widely used in bioconjugation, antibody-drug conjugates (ADCs) therapeutic, drug delivery and diagnostics field.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release ADC Has Curative Effect On Cell Surface Protein In Neuroblastoma here
News-ID: 2423563 • Views: …
More Releases from Biochempeg Scientific Inc.
Biopharma PEG Provides Multi-arm PEG Derivatives Crosslinked Into Hydrogels
Polyethylene (ethylene glycol) is a hydrophilic polymer that can have a very high water content when cross-linked into a network. Polyethylene glycol (PEG) is a suitable material for biological applications because it does not normally elicit an immune response. Since the 1970s, PEG has been used to modify therapeutic proteins and peptides in order to increase their solubility, reduce their toxicity, and prolong their cyclic half-lives. In the late 1970s,…

Semaglutide VS Liraglutide: Which Is Better For Weight Loss
Among adults with overweight or obesity, once-weekly subcutaneous semaglutide plus counseling for diet and physical activity results in significantly greater weight loss at 68 weeks than once-daily subcutaneous liraglutide, according to a study published in the Jan. 11 issue of the Journal of the American Medical Association.
Domenica M. Rubino, M.D., from the Washington Center for Weight Management and Research in Arlington, Virginia, and colleagues compared the efficacy and adverse event…
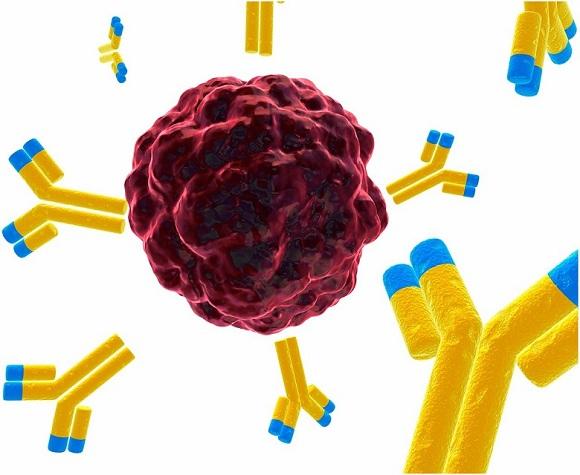
What Are Components And Mechanism Action of Antibody-Drug Conjugates
Antibody-Drug Conjugates (ADC) is a type of anticancer drug that links a drug to cancer-targeting antibodies. The antibody detects tumor-specific proteins expressed on cancer cells and directs the cytotoxic anticancer drug towards the cancer cell.
Compared to alternative cancer treatments, for example, chemotherapy, immunotherapy, radiation, and stem cell therapy, ADC combines chemotherapy and immunotherapy and allows selective delivery of anticancer drugs and reduces systemic exposure and toxicity of anticancer drugs.
Components of ADC
One…
Sacituzumab govitecan shows promise in treating the most aggressive type of brea …
A unique antibody drug conjugate (ADC), which delivers a high dose of a cancer-killing drug to tumor cells through a targeted antibody, has been found in a global phase 3 clinical study to nearly double the survival time of patients with refractory metastatic triple-negative breast cancer. The study of the ADC drug sacituzumab govitecan (SG), for which Massachusetts General Hospital (MGH) was a lead clinical research site after serving as…
More Releases for ALK
ALK ELISA Kits Market Outlook and Future Projections for 2030
The alk elisa kits market represents a dynamic and continually evolving landscape, shaped by changing consumer demands and technological advancements. In this comprehensive report, we provide an in-depth exploration of the market, designed for a wide range of stakeholders including manufacturers, suppliers, distributors, and investors. Our goal is to equip industry participants with essential insights that enable informed decision-making in an ever-changing market environment. This analysis not only examines the…
ALK-1 Antibody Market Outlook and Future Projections for 2030
The alk-1 antibody market represents a dynamic and continually evolving landscape, shaped by changing consumer demands and technological advancements. In this comprehensive report, we provide an in-depth exploration of the market, designed for a wide range of stakeholders including manufacturers, suppliers, distributors, and investors. Our goal is to equip industry participants with essential insights that enable informed decision-making in an ever-changing market environment. This analysis not only examines the current…
ALK-positive Non-Small Cell Lung Cancer Pipeline: Companies Advancing Targeted T …
The therapeutic landscape for ALK-positive Non-Small Cell Lung Cancer (ALK+ NSCLC), a genetically defined subset of lung cancer driven by ALK gene rearrangements, is undergoing a significant transformation. Biopharmaceutical companies are shifting focus from traditional chemotherapy to precision medicine approaches that directly inhibit ALK-driven oncogenic signaling. With multiple generations of ALK inhibitors now available and resistance mechanisms emerging, the spotlight is on next-generation inhibitors, combination regimens, and CNS-penetrant therapies designed…
Allergy Shots Market | ALK Abello, Aimmune Therapeutics, Allergy Therapeutics, A …
The global allergy shots market report is a comprehensive report that provides a detailed analysis of the current status and future trends of the allergy shots market worldwide. This report provides valuable information to industry stakeholders by offering an in-depth perspective on market dynamics, competitive landscape, growth opportunities, and key challenges faced by industry participants.
From the perspective of market dynamics, this report explores the factors driving the growth of the…
Global ALK Tyrosine Kinase Inhibitors (ALK-TKIs) Market Analysis By Major Manufa …
Global ALK Tyrosine Kinase Inhibitors (ALK-TKIs) Market: Driven factors and Restrictions factors
The research report encompasses a comprehensive analysis of the factors that affect the growth of the market. It includes an evaluation of trends, restraints, and drivers that influence the market positively or negatively. The report also outlines the potential impact of different segments and applications on the market in the future. The information presented is based on historical milestones…
ALK Tyrosine Kinase Inhibitors (ALK-TKIs) Market 2023: Analysis By Regional Outl …
Los Angeles, United State: The latest report published by QY Research presents a thorough analysis of the global ALK Tyrosine Kinase Inhibitors (ALK-TKIs) market. The research report evaluates the ever-changing market dynamics that are expected to impact the trajectory of the overall market. Analysts studied the historical achievements of the market and compared it to the current market trends, to chart the trajectory. For a detailed discussion about the global ALK Tyrosine…