Press release
Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market is Anticipated to Register a of CAGR of 3.9% During the Forecast Period (2020-2026)
Helicobacter Pylori infections, or H. Pylori, are the most common types of infections affecting the digestive tract. The infection is prevalent among half of the world’s population, who typically reside in unhygienic and poor sanitary conditions. It is the most common reason for dyspepsia, peptic ulcers and gastric adenocarcinoma.Click HERE to Get Synopsis of the Report- https://www.factmr.com/report/4774/helicobacter-pylori-hpylori-noninvasive-testing-market
In view of this, the demand for H. Pylori testing has increased, particularly non-invasive molecular techniques such as real-time PCR and Chemiluminescence Immunoassay (CLIA). The Helicobacter Pylori Non-Invasive Testing market is anticipated to register a moderate CAGR of 3.9% during the forecast period (2020-2026).
Key Takeaways of Global H. Pylori Non-Invasive Testing Market Study:
By test type, the urea breath test segment is anticipated to hold a major chunk of the market, amounting to over two-fifth. High rate of accuracy in detecting the presence of H. Pylori and usefulness in evaluating post-treatment status are poised to leverage this segment.
The Stool Antigen Test segment shall comprise the 2nd largest share of the global H. Pylori Non-invasive testing market, accounting for over 40%. Simplicity and accuracy of the test in determining infection is anticipated to drive the segment’s growth.
By test method, the Laboratory-based Tests segment shall generate high demand. The ability to test a large number of patients and their accurate and precise methodologies are anticipated to be the key drivers. The segment is foreseen to hold more than three-fifth of the market.
For detailed insights on enhancing your product footprint, request for a sample here- https://www.factmr.com/connectus/sample?flag=S&rep_id=4774
By end-use, diagnostic labs are anticipated to be the most lucrative, capturing almost half of the total market. Key pharmaceutical companies partnering to provide wider reach for testing H. Pylori are anticipated to drive the market. For instance, in 2010, Meridian Bioscience entered into an agreement with DiaSorin S.p.A to develop infectious disease tests for use on its LIAISON automated instrumets.
North America is anticipated to be the most promising market. This is attributed to changing healthcare reforms. These reforms have directed organizations to abandon serology tests due to its uncertainty in predicting infections and to switch to urea-based and stool antigen testing. The region is anticipated to hold 31.1% of the H. Pylori testing market.
Asia-Pacific shows modest growth opportunities, owing to increased awareness about H. Pylori infections and government efforts to contain the risk of infections. Moreover, H. Pylori testing among communities with gastric cancer has increased, preventing further risks. The region is anticipated to expand modestly at a CAGR of 4.5%.
“The global helicobacter pylori non-invasive testing market shows modest future growth prospects. The increasing prevalence of h. pylori associated diseases have prompted manufactures to invent non-invasive testing and treatment methods to reduce the incidence of such diseases,” concludes a Fact.MR analyst.
Need more information about Report Methodology? Click here- https://www.factmr.com/connectus/sample?flag=RM&rep_id=4774
Global Helicobacter Pylori Non-Invasive Testing Market: Competitive Landscape:
The global H. Pylori non-invasive testing market comprises of the following market players: Meridian Bioscience, Inc., DiaSorin S.p.A, Alere, Sekiusi Diagnostics and Biomerica, Inc. Besides these, a host of other players have a significant market presence as well. These are Biohit Oyj, Exalenz Bioscience Ltd., Aalto Bio Reagents Ltd., Certest Biotec S.L. and CorisBioconcept SPRL.
Meridian Bioscience, Inc. markets its tests and assay kits to hospitals, reference laboratories and other diagnostic manufacturers in more than 60 countries. Its H. Pylori testing products include the Premium Platinum HpSA PLUS or Premium H. Pylori. It is an in vitro qualitative procedure to detect the presence of H. Pylori bacteria in the human stool.
Exalenz Biosciences manufactures the H. Pylori IgG ELISA Kit, BreathID HP, BreathID Smart and Breath ID Lab devices. These devices conduct a quick and accurate Urea Breath Test for diagnosing H. Pylori infection and confirming post-treatment eradication. The test is conducted in real-time by continuously sampling breath, eliminating the need for sample collection, labeling, transport and analysis.
COVID-19 Market Insights:
Although there is a dearth of evidence suggesting any links between the COVID-19 and H. Pylori infections, experts have concluded that patients need to be provided treatment on a priority basis. This is because the COVID-19 infection affects the gastrointestinal tract, inducing nausea, vomiting and diarrhea. Since H. Pylori primarily infects the GI tract, it is important for those patients to be provided immediate medical attention if infected with the COVID-19.
As the pandemic is spreading at an exponential rate, key manufacturers are accelerating their efforts to contain its spread. This involves redirecting their resources towards developing COVID-19 testing kits. For instance, Meridian Bioscience has launched the QuickPac II 2019 COVID-19 IgG and IgM qualitative testing kit for the detection of potential antibodies to combat the novel coronavirus strain in the human serum, plasma or blood. Similarly, DiaSorin S.p.A announced the launch of its LIAISON SARS CoV-2 S1/S2 IgG serological test. Such developments are anticipated to shift focus away from H. Pylori testing, leading to a dip in its demand. However, patients requiring urgent care will be provided with the appropriate treatment.
For in-depth competitive analysis, buy now- https://www.factmr.com/checkout/4774/S
Looking for more information?
The research study on the global helicobacter pyroli (h. pyroli) non-invasive testing market by Fact.MR incorporates an unbiased assessment of key factors and trends responsible for shaping the landscape of the global helicobacter pyroli (h. pyroli) non-invasive treatment market over 2020-2026. It includes a detailed assessment of key parameters that are anticipated to exert influence during 2020-2026. Market statistics have been presented on the basis of Non-invasive Test Type (Serology Test, Stool Antigen Test and Urea Breath Test), Test Method (Laboratory Based Tests and Point-of-Care Tests) and End User (Hospitals, Diagnostic Laboratories and Clinics) across five major regions.
US Sales Office:
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
Corporate Headquarter:
Unit No: AU-01-H Gold Tower (AU),
Plot No: JLT-PH1-I3A,
Jumeirah Lakes Towers,
Dubai, United Arab Emirates
Email : sales@factmr.com
Website : https://www.factmr.com
Research Insight: https://www.factmr.com/report/4774/helicobacter-pylori-hpylori-noninvasive-testing-market
Content Source: https://www.factmr.com/media-release/1538/global-helicobacter-pylori-hpylori-noninvasive-testing-market
About Fact.MR
Market research and consulting agency with a difference! That’s why 80% of Fortune 1,000 companies trust us for making their most critical decisions.
We have offices in US and Dublin, whereas our global headquarter is in Dubai.While our experienced consultants employ the latest technologies to extract hard-to-find insights, we believe our USP is the trust clients have on our expertise.
Spanning a wide range – from automotive & industry 4.0 to healthcare & retail, our coverage is expansive, but we ensure even the most niche categories are analyzed.
Reach out to us with your goals, and we’ll be an able research partner.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market is Anticipated to Register a of CAGR of 3.9% During the Forecast Period (2020-2026) here
News-ID: 2267999 • Views: …
More Releases from Fact.MR
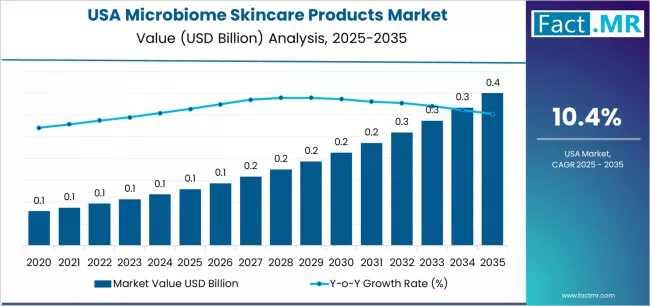
USA Demand for Microbiome Skincare Products in USA Outlook 2025-2035: Key Develo …
The U.S. microbiome skincare market is projected to experience rapid, data-driven growth over the next decade, driven by rising consumer awareness, ingredient innovation, and the integration of personalized diagnostics. Analysts estimate the U.S. microbiome skincare segment at approximately USD 0.13 billion in 2025, with a projected increase to USD 0.35 billion by 2035, representing a compound annual growth rate (CAGR) of 10.4%.
To access the complete data tables and in-depth insights,…
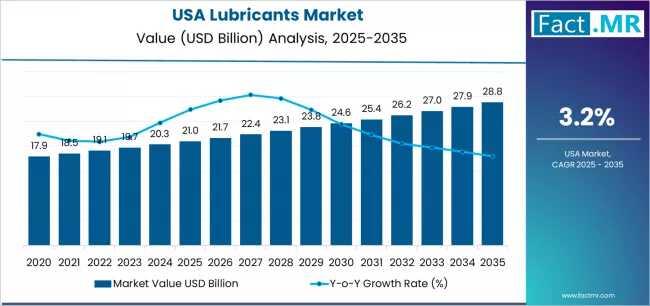
USA Demand for Lubricants in USA Outlook 2025-2035: Key Developments and Future …
The U.S. lubricants market is projected to experience steady growth through 2035, driven by shifts in mobility, industrial demand, and sustainability trends. The market was valued at approximately $41.2 billion in 2024 and is expected to grow at a compound annual growth rate (CAGR) of 2.5%, reaching $52 billion by 2035.
To access the complete data tables and in-depth insights, request a Discount On The Report here: https://www.factmr.com/connectus/sample?flag=S&rep_id=12463
…
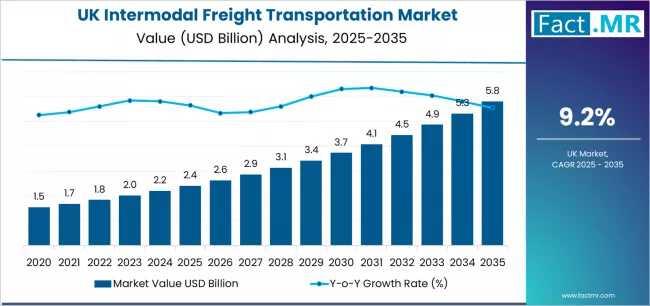
European Demand for Intermodal Freight Transportation in UK Outlook 2025-2035: K …
"Demand for intermodal freight transport connecting the UK and continental Europe is projected to grow at a CAGR of 6.8% between 2025 and 2035, driven by rising trade volumes, decarbonization mandates, and digital logistics innovations. The intermodal market, encompassing rail, short-sea shipping, and last-mile road delivery, is expected to handle over 18 million TEUs (twenty-foot equivalent units) annually by 2035, up from 10.2 million TEUs in 2025.
To access the complete…
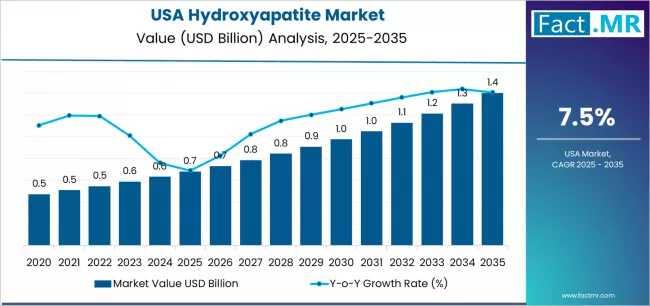
USA Demand for Hydroxyapatite in USA Outlook 2025-2035: Key Developments and Fut …
The United States hydroxyapatite (HAp) market is projected to experience sustained, data-driven growth from 2025 through 2035, fueled by rising clinical demand, technological innovation, and expanded applications in regenerative medicine. Market modeling indicates that U.S. consumption of hydroxyapatite will nearly double over the next decade, with strong adoption in orthopedic, dental, and advanced biomaterial sectors.
To access the complete data tables and in-depth insights, request a Discount On The Report here:…
More Releases for Pylori
Helicobacter Pylori (H. Pylori) Market Trends, Treatment Landscape, and Forecast
Helicobacter pylori (H. pylori) is a gram-negative bacterium that infects the stomach lining, leading to a variety of gastrointestinal disorders, including peptic ulcers, chronic gastritis, and gastric cancer. It is estimated that more than half of the world's population is infected with H. pylori, although many people remain asymptomatic. When symptomatic, H. pylori infections can cause stomach pain, bloating, nausea, and more severe complications like bleeding ulcers or gastric cancer.…
Helicobacter Pylori (H. Pylori) Non-invasive Testing Market Is Booming So Rapidl …
HTF MI just released the Global Helicobacter Pylori (H. Pylori) Non-invasive Testing Market Study, a comprehensive analysis of the market that spans more than 143+ pages and describes the product and industry scope as well as the market prognosis and status for 2025-2032. The marketization process is being accelerated by the market study's segmentation by important regions. The market is currently expanding its reach.
Key Players in This Report Include:
Abbott Laboratories,…
Helicobacter pylori (H. pylori) Infections Market is expected to reach $9.1 bill …
Helicobacter pylori (H. pylori) is a spiral-shaped bacterium that colonizes the stomach lining, causing chronic gastritis, peptic ulcers, and significantly increasing the risk of gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma. It affects nearly half of the global population, though prevalence varies by geography, socioeconomic conditions, and sanitation levels.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/71739
Management of H. pylori infections typically relies on antibiotic-based eradication therapies, combined…
Helicobacter pylori H. pylori Diagnostics Market Is Set to Experience Revolution …
The Helicobacter pylori (H. pylori) diagnostics Market was valued at USD 554.46 million in 2021. It is expected to increase from USD 587.68 million in 2022 to USD 953.25 million by 2030, with a compound annual growth rate (CAGR) of 6.01% during the forecast period of 2023-2030.The popularity of H. pylori diagnostics is growing due to its benefits in improving diagnosis, enabling effective treatment, and reducing healthcare costs.
Detecting H. pylori…
Helicobacter Pylori (H Pylori) Non-Invasive Testing Market Share, Size, Demand, …
Helicobacter Pylori (H Pylori) Non-Invasive Testing Market size was valued at over USD 447 million in 2022. Driven by the increasing instances of peptic ulcers, the market is slated to register over 6.5% CAGR from 2023 to 2032. The Helicobacter Pylori (H. Pylori) Non-invasive Testing market report is a perfect foundation for people looking out for a comprehensive study and analysis of the Helicobacter Pylori (H. Pylori) Non-invasive Testing market.…
Helicobacter pylori (H. pylori) Diagnostics Market to Witness Exponential Growth …
Helicobacter pylori (H. pylori) is a spiral shaped gram-negative aerobic bacterium commonly found in the stomach. Bacteria plays a vital role in balancing stomach ecology. When H. pylori invades and attacks the stomach lining, it causes stomach infections. These infections are commonly found in both men as well as women. Nearly 30% of the world's population is infected by H. pylori. In some countries, the bacteria infects more than 50%…