Press release
Pharmaceutical contract manufacturing and API sourcing: why not Central Europe?
At present, only a few companies based in Central Europe and the Balkans emphasise their contract manufacturing and API sourcing services. There is relatively strong competition from companies from the Far East and from Western Europe.On the other hand, the potential of the region seems to be considerable, with Poland and the Czech Republic still the most attractive locations, according to the latest report from PMR, a research and consulting company, entitled "Pharmaceutical contract manufacturing and API sourcing in Central Europe and the Balkan states 2011".
Higher production costs matched by higher quality
Although companies in CE and the Balkans are usually unable to compete with Indian and Chinese manufacturers, because of the production costs, there are several regional advantages over the Far East; these include:
Legislation based on EU standards or, in Serbia and Croatia, laws which are being harmonised with those of the EU, with the aim of joining.
Relatively wide application of ISO and GMP standards.
Most CE companies can cooperate within the tariff-free trade framework of the EU.
A rapidly developing biomanufacturing industry.
"It must be admitted, however, that there is a much more limited choice of companies which offer contract manufacturing services and/or API in CE and the Balkans in comparison with India and China" says Agnieszka Skonieczna, PMR Senior Pharmaceutical Market Analyst and the report co-author.
According to the European Directorate for the Quality of Medicines & Healthcare (EDQM), India and China are also progressing as non-European users of EDQM Reference Standards. In May and September 2010, two conferences were organised in cooperation with the EDQM in India and China on the quality of APIs. These highlighted important matters relevant to the quality of APIs, dossier assessment, inspections and pharmacopoeial requirements.
Poland and the Czech Republic are the most attractive locations
PMR analysis suggests that the Central European and Balkan countries offer the opportunity to manufacture not only APIs, other specialist chemicals and plant raw materials, but also various finished dosage forms of pharmaceuticals, dietary supplements, semi-finished products and biotechnology products. In the area in question, the most attractive countries for pharmaceutical contract manufacturing and API sourcing are Poland and the Czech Republic. There are several dozen companies in both countries which offer such services.
There are numerous companies which specialise in API production in the Czech Republic in comparison with the other countries in the region and, in fact, the country might be thought of as a centre of API production in terms of Central Europe as a whole. Contract manufacturing services pertaining to finished dosage forms in the country focus primarily on the production of dietary supplements. This is associated with a long tradition of dietary supplement production in the Czech Republic.
In Poland there are not many API producers at present, and most companies do not produce APIs for their own needs. With regard to contract manufacturing, there are several Polish companies with a long tradition of pharmaceutical manufacturing which also engage in production under contract. There are also many companies which specialise in the production of dietary supplements and/or essentially natural raw materials used in the production of pharmaceuticals, dietary supplements and cosmetics.
EU to combat inferior APIs
Because of the questionable quality of some APIs exported into the European Union, in April 2010 the European Commission proposed that the drug regulator in any country which exports its APIs to the European Union should carry out regular inspections at the API supplier’s manufacturing facility to ensure that the company is not violating GMP standards and present a written declaration confirming product quality. If such regulations come into force, they may weaken the position of API manufacturers outside the EU. For example, Indian API manufacturers fear that the procedure could lead to unnecessary delays in export consignments because they would have a long wait before being cleared by the Indian authorities. At present, API suppliers to the EU need only obtain a Certificate of Suitability of European Pharmacopoeia monographs (CEPs) issued by the European Directorate for the Quality of Medicines & Healthcare.
In its 2015 Roadmap, the draft of which was published in January 2011, the European Medicines Agency highlighted the quality of APIs manufactured outside the EU as an area of concern, because the international sourcing of active pharmaceutical ingredients makes it possible for substandard material to enter the supply chain.
Will contract manufacturing become the norm?
The substantial changes seen in manufacturing infrastructure began only a few years ago, and the global contract manufacturing market, including the Central Europe area, will still be growing rapidly in subsequent years, according to PMR.
Market growth will be accelerated by the expiry of the patents on several blockbusters in the next few years. This will compel large multinational companies, whose revenues are expected to decline significantly, to look for savings. One of the solutions could be the outsourcing of pharmaceutical production.
Companies may also be compelled to reduce their costs because of uncongenial legislation introduced by governments. This includes, for example, the Reimbursement Act in Poland, which will require pharmaceutical companies to take part in a payback mechanism (and to participate in NFZ reimbursement costs). It also introduces fixed margins and prices for reimbursed products. In Hungary the government has decided to increase the tax payable by pharmaceutical manufacturers on revenues from reimbursed drugs from 12% to 20% from 1 July 2011 onwards.
This press release is based on information contained in the latest PMR report entitled "Pharmaceutical contract manufacturing and API sourcing in Central Europe and the Balkan states 2011" (http://www.pmrpublications.com/online_shop/Pharmaceutical-contract-manufacturing-API-sourcing-Central-Europe-2011.shtml
).
For more information on the report please contact:
Marketing Department:
tel. /48/ 12 618 90 00
e-mail: marketing@pmrcorporate.com
PMR permits the republishing of this press release in part or in whole provided that all portions of text, graphics, diagrams and tables identify PMR in a proper citation format: "Source: PMR". All citations should be accurate and quoted without manipulation and must not be used out of context.
For more information about citing PMR, please consult our Citation Policy.
PMR (www.pmrcorporate.com) is a British-American company providing market information, advice and services to international businesses interested in Central and Eastern European countries as well as other emerging markets. PMR's key areas of operation include business publications (through PMR Publications), consultancy (through PMR Consulting) and market research (through PMR Research). Being present on the market since 1995, employing highly skilled staff, offering high international standards in projects and publications, providing one of most frequently visited and top-ranked websites, PMR is one of the largest companies of its type in the region.
PMR Ltd. Sp. z o.o.
Dekerta 24, 30-703 Kraków, Poland
tel. /48/ 12 618 90 00, fax /48/ 12 618 90 08
www.pmrcorporate.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmaceutical contract manufacturing and API sourcing: why not Central Europe? here
News-ID: 188197 • Views: …
More Releases from PMR Ltd.

Roads to be a driving force of the Bulgarian construction market
Access to EU funding and the investments in the energy sector are the predominant factors determining the development of the Bulgarian civil engineering construction sector in the next three years. It is expected that this segment will lead the development of the construction market in Bulgaria.
According to the research company PMR’s latest report, entitled “Construction sector in Bulgaria 2012 – Development forecasts for 2012-2014”, the absorption rate of the programmes…
Polish road construction market to grow nearly 25% in 2011
Activity in Polish road construction will peak in 2011 and 2012.
Driven by substantial capital expenditure by the General Directorate for National Roads and Motorways (GDDKiA), the value of roadwork will exceed PLN 30bn (€7.5bn) already in 2011. Delays in the implementation of projects related to Euro 2012 and sustained investments in local roads will prepare the road market for the anticipated period of reduced spending on the construction of…
Construction industry in Romania expected to stabilise in 2011
Prior to the start of the crisis, the Romanian construction industry was one of the most vibrant in the European Union.
This changed dramatically in 2009, when construction output fell by 15%, with a similar reduction following in 2010. The current year should finally lead to a measure of stabilisation on the market, but growth is not likely to return before 2012.
Romania was the region's best performer in construction a…
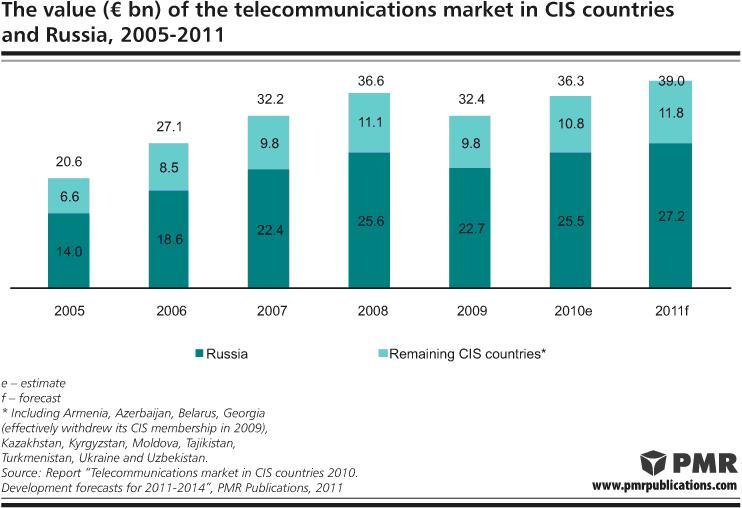
Telecoms market in CIS region to reach €39bn in 2011
The CIS telecommunications market is recovering after the crisis period. In euro terms, the market closed 2010 with double-digit growth, chiefly due to the improvement of macroeconomic indicators and the gradual strengthening of local currencies. This year, the market is also expected to increase fuelled by further development of broadband services.
Russia leading
The telecommunications market in Russia is by far the largest among all CIS countries. Revenues generated only on the…
More Releases for API
API Management Market Size, Trends Analysis 2032 by Key Vendors- Google, Cloud A …
USA, New Jersey: According to Verified Market Research analysis, the global API Management Market size was valued at USD 4.37 Billion in 2024 and is projected to reach USD 33.07 Billion by 2032, growing at a CAGR of 28.77% from 2026 to 2032.
What is the current outlook of the API Management Market and its expected growth potential?
The API Management Market is witnessing robust expansion due to the growing need…
Api 607 Vs API 608: A Comprehensive Comparison Guide Of Industrial Valve
Introduction: Why are API standards so important for industrial valves?
In high-risk industries such as oil and gas, chemicals and power, the safety and reliability of valves can directly affect the stability of production systems. The standards set by API (American Petroleum Institute) are the technical bible of industrial valves around the world. Among them, API 607 and API 608 are key specifications frequently cited by engineers and buyers.
This article will…
Vehicle API Market 2023 | Futuristic Technology- CarAPI, Caruso, One Auto API, A …
The Vehicle API market research report delivers accurate data and innovative corporate analysis, helping organizations of all sizes make appropriate decisions. The Vehicle API report also incorporates the current and future global market outlook in the emerging and developed markets. Moreover, the report also investigates regions/countries expected to witness the fastest growth rates during the forecast period.
The Vehicle API research report also provides insights of different regions that are…
Face Recognition API Market Growth, Business Overview 2023, and Forecast to 2030 …
Facial recognition is a way of recognizing a human face through technology. A facial detection system uses biometrics to map facial features from a photograph or video. It compares information with a database of known faces to find a match. Moreover, the accuracy of facial recognition systems has improved way better in the last decade. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger,…
API Management Market Report 2018: Segmentation by Solution (API Portal, API Gat …
Global API Management market research report provides company profile for Akana, Inc. (U.S.), Apiary, Inc. (U.S.), Axway, Inc. (France), CA Technologies, Inc. (U.S.), Cloud Elements, Inc. (U.S.), Dell Boomi, Inc. (U.S.), DigitalML (U.S.), Fiorano Software, Inc. (U.S.), Google, Inc. (U.S.), Hewlett-Packard Enterprises Co. (U.S.), IBM Corporation (U.S.), Mashape Inc. (U.S.) and Others.
This market study includes data about consumer perspective, comprehensive analysis, statistics, market share, company performances (Stocks), historical…
Telecom API Market: OTT Service Providers Continue Cutting into Telecom API Prof …
The highly fragmented market of telecom API holds a staggering number of service providers and aggregators that are already offering their APIs to various telecom carriers. Alcatel Lucent, Apigee Corp., and Fortumo OU were the leading providers of telecom API from a global perspective in 2014. Telecom carriers have partnered with them and other prominent players in the past to launch APIs in the market.
According to Transparency Market Research’s latest…