Press release
Key Emerging Trends in Diabetes Drugs Discussed in a New Market Research Report
Rising urbanization, increasing intake of sugary foods, and changing lifestyle preferences like lack of exercise are leading to growth in obesity globally. Earlier, developed markets in US and Europe faced a strong demand for diabetes drugs due to the large number of patients.However, today these patterns are a common sight in developing economies of Asia as well. For example, India has become home to one of the largest diabetic population in the world. This population counted in millions, forms over 8% of India’s total population.
Obtain Report Details @ https://www.transparencymarketresearch.com/diabetes-drug-market.html
FDA Approval to Boost the Diabetes Drugs Market
FDA or the US Food and Drug Administration approved a non-insulin drug for pediatric patients in 2019.
The drug, Victoza has shown efficacy in treating type 2 Diabetes. The drug is behind metformin, which was the first drug to be approved for pediatric patients in 2000.
Victoza has been under the trial since 2010. According to a statement released by FDA, increasing number of children is being diagnosed with the type 2 Diabetes. This prompted the FDA to expand an additional treatment option.
According to Center for Disease Control and Prevention, there are additional 5000 deaths in the US each year among the young population.
Get Sample with Latest Research @ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=14672
Technological Advancement Promise New Opportunities
Currently, the pace of technological innovation is leading to growth in many sectors. The medical sector, consisting of pharmaceutical companies and insurance companies are major beneficiaries of this development.
On one hand, technologies like 3D printing, growing sources of outsourcing are leading to major reductions in costs. Additionally, growing support for innovation in major economies like US and China are spelling a boon for the industry.
Moreover, portable monitoring technologies available through smartphones are making way for better healthcare systems and leading to more preventive care for the industry.
This has led to an increase in number of diagnosis. On the other hand, it has helped the insurance support a more solid preventive care framework for the treatment of various diabetics.
Contact Us
Transparency Market Research
State Tower,
90 State Street, Suite 700
Albany, NY 12207
United States
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: http://www.transparencymarketresearch.com
About Us
Transparency Market Research is a next-generation market intelligence provider, offering fact-based solutions to business leaders, consultants, and strategy professionals.
Our reports are single-point solutions for businesses to grow, evolve, and mature. Our real-time data collection methods along with ability to track more than one million high growth niche products are aligned with your aims. The detailed and proprietary statistical models used by our analysts offer insights for making right decision in the shortest span of time. For organizations that require specific but comprehensive information we offer customized solutions through adhoc reports. These requests are delivered with the perfect combination of right sense of fact-oriented problem solving methodologies and leveraging existing data repositories.
TMR believes that unison of solutions for clients-specific problems with right methodology of research is the key to help enterprises reach right decision.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Key Emerging Trends in Diabetes Drugs Discussed in a New Market Research Report here
News-ID: 1862336 • Views: …
More Releases from Transparency Market Research
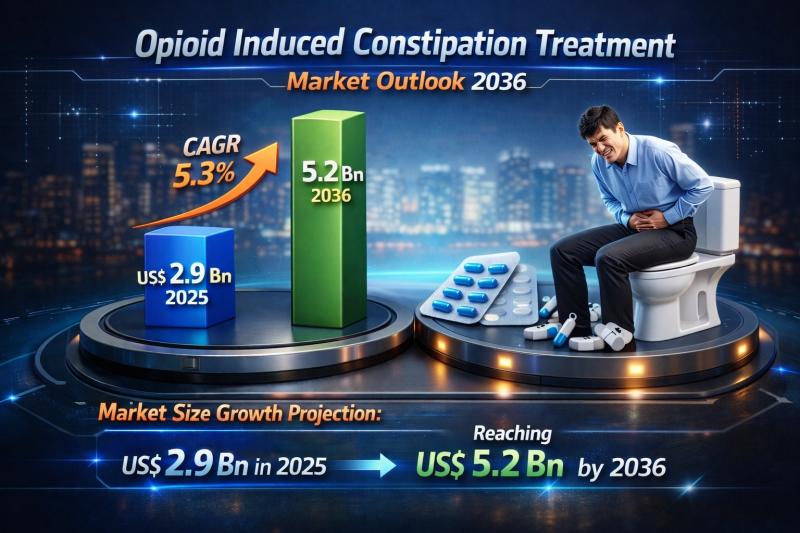
Global Opioid Induced Constipation Treatment Market Set to Reach USD 5.2 Billion …
The global opioid induced constipation (OIC) treatment market is witnessing steady and sustained growth as healthcare systems worldwide place increasing emphasis on comprehensive pain management and supportive care. Valued at US$ 2.9 billion in 2025, the market is projected to reach US$ 5.2 billion by 2036, expanding at a compound annual growth rate (CAGR) of 5.3% from 2026 to 2036. Growth is primarily fueled by the rising prevalence of chronic…
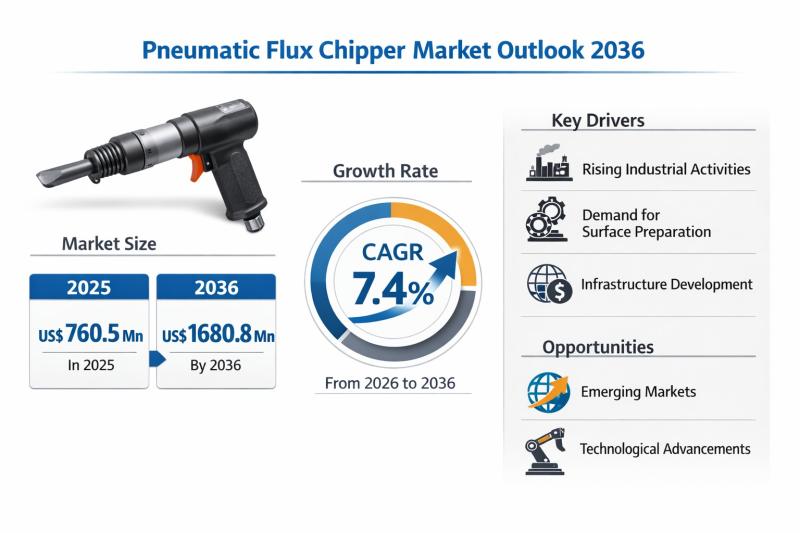
Pneumatic Flux Chipper Market Expanding at 7.4% CAGR Through 2036 - By Product T …
The global Pneumatic Flux Chipper Market is set to witness sustained and resilient growth over the next decade, underpinned by expanding heavy manufacturing activities, rising welding and fabrication demand, and continuous investments in industrial infrastructure across emerging and developed economies. According to the latest industry analysis, the market was valued at US$ 760.5 Mn in 2025 and is projected to reach US$ 1,680.8 Mn by 2036, expanding at a compound…

AI in Automotive Market Outlook 2036: Global Industry to Surge from US$ 19.8 Bil …
The AI in automotive market is entering a phase of exponential expansion, supported by rapid digitization of vehicles, growing safety mandates, and consumer demand for intelligent mobility. The global market was valued at US$ 19.8 Bn in 2025 and is projected to reach US$ 244.4 Bn by 2036, registering a remarkable CAGR of 27% from 2026 to 2036.
This growth trajectory reflects the transition of automobiles from mechanically driven products to…
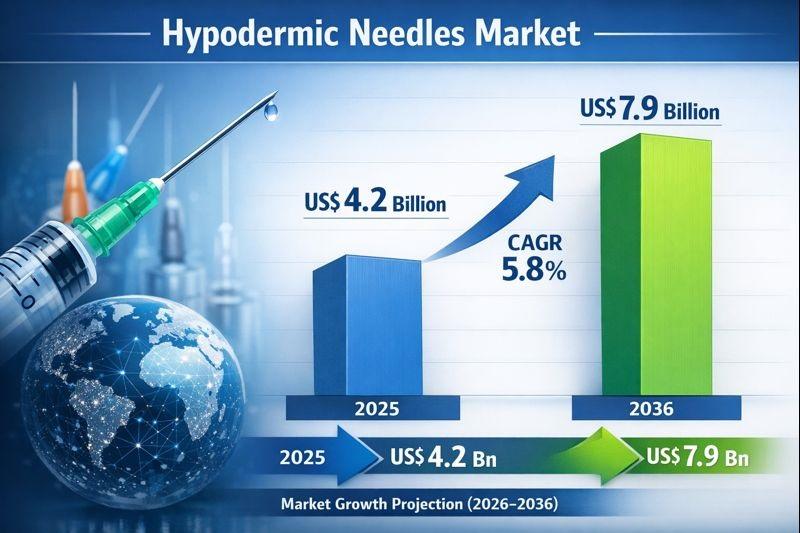
Hypodermic Needles Market to Reach US$ 7.9 Billion by 2036 on Rising Injectable …
The global hypodermic needles market was valued at approximately US$ 4.2 billion in 2025 and is projected to reach around US$ 7.9 billion by 2036, expanding at a CAGR of nearly 5.8% from 2026 to 2036, driven by the rising prevalence of diabetes, cancer, and chronic diseases, growing demand for injectable drugs and biologics, and the expansion of global vaccination and immunization programs; increasing adoption of safety-engineered and disposable needles,…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…