Press release
Home Diagnostics Market Sales Likely to Surge at Exceptional Rate during Forecast Period,2018 to 2026 | Key Players - Abbott Laboratories, ACON Laboratories , BTNX Inc., ARKRAY , Assure Tech (Hangzhou) co. , Becton Dickinson & Company, Bionime Corporation
Fact.MR, in its latest market study, opines that the home diagnostics market will grow at a healthy 3.5% value CAGR, surpassing US$ 6.3 million by 2026. According to the report, sales will remain buoyant in developed regions, with North America and Europe collectively accounting for over 50% revenue share of the market. The Fact.MR study remains bullish on the prospects of home diagnostics market players in developing countries, as growing focus on health & wellness, and emergence of many Asian countries as medical tourism hubs creates opportunities.Request 100 Page Sample Report Now: https://www.factmr.com/connectus/sample?flag=S&rep_id=1892
Increasing preference for self-diagnosis continues to be a trigger for home diagnostics sales, whereas a combination of industry-specific and regulatory factors continue to influence the strategies of market players. Governing bodies such as the U.S. Food and Drug Administration (FDA) and the European Commission regulate home diagnostics with stringent quality control regulations and labeling requirements. Gaining EU CE mark, FDA 510(k) clearance, and FDA approved Premarket Approval Application (PMA) continues to be a primary focus for home diagnostics manufacturers who are looking to scale up their presence in developed markets. For instance, Abbott Laboratories, a leading pharmaceutical company in the U.S., recently got an FDA approval for marketing its FreeStyle® Libre 14 day Flash Glucose Monitoring system in the U.S. ACON Laboratories, another prominent player in the home diagnostics market announced that it has received FDA clearance of Mission® U120 urine reagent strips. According to the report, compliance to ever-evolving regulations and gaining regulatory approval remains both an opportunity and challenge for market players.
The report also finds that home diagnostics manufacturers are focusing on incorporating advanced technology to differentiate their offerings in the market. For example, Abbott Laboratories adopted next-generation sensor technologies to upgrade its FreeStyle Libre system (10 day) to the FreeStyle® Libre 14 day Flash Glucose Monitoring system with improved accuracy and better mean absolute relative difference.
To know the latest trends and insights prevalent in this market, click @ https://www.factmr.com/report/1892/home-diagnostics-market
The Fact.MR study finds that glucose monitoring devices will continue to witness robust demand and dominate the home diagnostics market with over than 80% revenue share in the market. According to the World Health Organization (WHO), the number of people suffering from diabetes reached 422 million in 2014, and it will soon become a leading cause of death. Increasing prevalence of diabetes worldwide and the growing need for monitoring sugar levels among diabetes patients is likely to boost adoption of glucose monitoring devices during the assessment period. Home diagnostics market players are aware of the surging demand for glucose monitoring devices, and are focusing on increasing their product portfolio in this segment. The other lucrative segments in the home diagnostics market include pregnancy tests and ovulation prediction kits.
According to the report, although demand for home diagnostics is concentrated in developed countries, home diagnostics market players are putting efforts to boost uptake in developing regions. According to statistics published by WHO, the prevalence of diabetes has been the highest in middle and low-income countries.
Anticipating the burgeoning demand for glucose monitoring devices in emerging Asia, players in the home diagnostics market are shifting their focus towards developing nations, including China and India. ARKRAY, Inc. – a Japanese manufacturer in the home diagnostics market – recently announced that it will strengthen its diabetes testing instrument production capacity in China. Leading manufacturers in the home diagnostics market are modifying their marketing strategies to improve awareness about cost-effective and efficient home diagnostics to leverage lucrative opportunities in the Asia-Pacific region.
Ask Industry Expert about this Report - https://www.factmr.com/connectus/sample?flag=AE&rep_id=1892
Contact Us
Fact.MR
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Email: sales@factmr.com
Web: https://www.factmr.com/
Blog: https://factmrblog.com/
Read Industry News at - https://www.industrynewsanalysis.com/
About Fact.MR
Fact.MR is a fast-growing market research firm that offers the most comprehensive suite of syndicated and customized market research reports. We believe transformative intelligence can educate and inspire businesses to make smarter decisions. We know the limitations of the one-size-fits-all approach; that's why we publish multi-industry global, regional, and country-specific research reports.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Home Diagnostics Market Sales Likely to Surge at Exceptional Rate during Forecast Period,2018 to 2026 | Key Players - Abbott Laboratories, ACON Laboratories , BTNX Inc., ARKRAY , Assure Tech (Hangzhou) co. , Becton Dickinson & Company, Bionime Corporation here
News-ID: 1784055 • Views: …
More Releases from Fact.MR
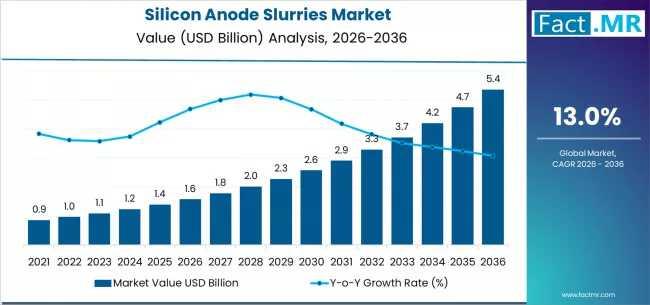
Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…

Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…
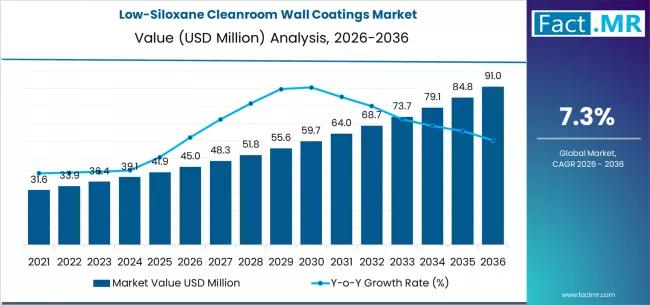
Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…

Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
