Press release
Bio-Gate clarifies FDA regulatory pathway for HyProtect™
Nuremberg, 29 January 2019 – Bio-Gate AG, concluded a pre-submission with FDA regarding its HyProtect™ coating technology, which is an ultrathin plasma coating containing pure silver and polysiloxane that protects the surfaces of medical implants against bacterial colonization.Via the FDA’s pre-submission process, Bio-Gate clarified the regulatory paths. For a certain range of orthopaedic products, a 510(k) pathway without human data is possible. Bio-Gate is in collaboration and communication with orthopaedic companies interested in the commercialization of the HyProtect™ technology in the US and European markets.
Located in Nuremberg, Germany, Bio-Gate AG is an ISO 13485 certified contract service provider for medical devices that include HyProtect™ and MicroSilver BG™ technologies, and the ISO 17025 certified subsidiary QualityLabs, a laboratory for biological and chemical testing.
Please contact for North America:
Karl F Richter
President
RFH BioTek Inc
27 Taylorwood Avenue,
Bolton, Ontario,
Canada L7E 1J5
Phone: +1-905-857-0219
Email: rfh@rfhbiotek.com
Please contact for Europe:
Thomas Konradt
Director Business Development
Bio-Gate AG
Neumeyerstrasse 28-34
90411 Nuremberg, Germany
Phone: +49 (0) 911 / 477523-108
Fax: +49 (0) 911 / 477523-101
e-mail: thomas.konradt@bio-gate.de
Disclaimer:
This publication constitutes neither an offer to sell nor an invitation to buy securities. The shares in Bio-Gate AG (the "Shares") may not be offered or sold in the United States or to or for the account or benefit of "U.S. persons" (as such term is defined in Regulation S under the U.S. Securities Act of 1933, as amended (the "Securities Act")). The securities have already been sold.
About Bio-Gate AG
The medical technology company Bio-Gate AG develops and markets applications which use unique silver technology to help prevent infections and thus to improve health. Bio-Gate AG’s specialty is using pure silver to treat materials and surfaces that are used in all areas of daily life – thus providing long-term and medically effective protection against bacteria, microorganisms and other pathogens. Bio-Gate AG works in three fields to supply a variety of products that provide antimicrobial protection: material enhancement, surface coating and testing the antimicrobial or anti-adhesive properties of materials. The Nuremberg-based company offers systems that stretch across the entire value-adding chain, from development to approval to production. For more information, please visit www.bio-gate.de.
Bio-Gate AG
Neumeyerstr. 28-34
D-90411 Nürnberg
www.bio-gate.de
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Bio-Gate clarifies FDA regulatory pathway for HyProtect™ here
News-ID: 1545346 • Views: …
More Releases from ins trade media service

Bio-Gate concludes another cooperation agreement with leading multinational impl …
Medical technology business further strengthened
Coating of orthopaedic revision implants with HyProtect technology
Focus on protection against infections with multi-resistant germs
Nuremberg/Bremen, 5 July 2022 - Bio-Gate AG (ISIN DE000BGAG981), a leading provider in the field of innovative health technologies, has concluded a contract with a leading multinational medical device manufacturer for the coating of orthopaedic implants in human medicine. As part of the cooperation and the joint project management, Bio-Gate is responsible…

Walki introduces a broad portfolio of recyclable materials for the growing froze …
Walki is answering to the growing demand for frozen food by expanding its portfolio of recyclable materials.
The global market for frozen food is expected to grow to 322 billion euros by 2026. Although the demand is growing globally across all age groups, it is especially popular among younger consumers.
"Frozen food is an ideal way to prolong shelf life without losing out on the vitamins. It is also an excellent way…
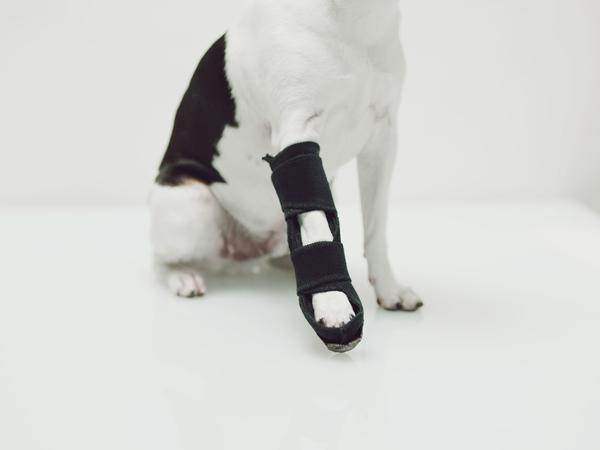
Dassiet and OrthoPets collaborate to provide safer and easier orthopedic care fo …
The teams combine Dassiet’s clinically proven medical and material innovations with OrthoPets' strong experience in custom pet orthoses to provide human level care for animals.
The material innovation company Dassiet, and the leading, US based pet orthosis manufacturer OrthoPets have started collaboration to create sustainable, safe and user-friendly pet healthcare products. Both companies want to first tackle the issues surrounding veterinary casting, splints, and bandages, and to provide vets, pets and…
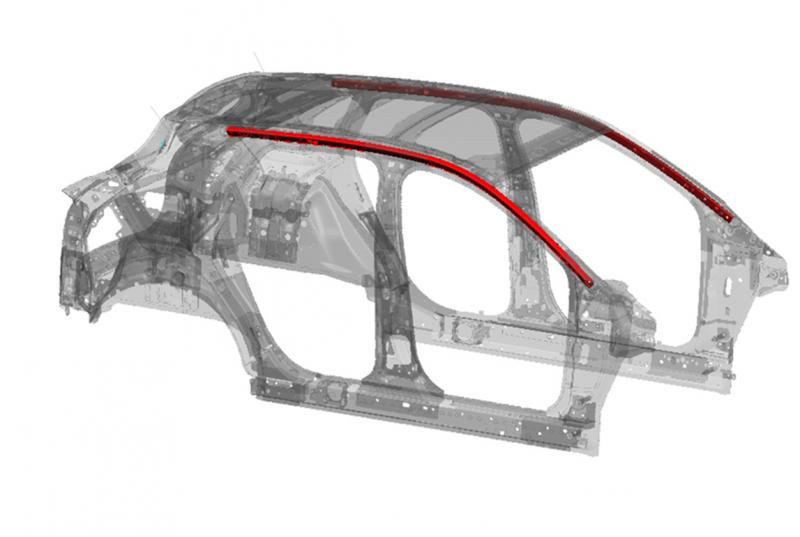
Shape Corp. wins Swedish Steel Prize 2019
The winner of the 20th international Swedish Steel Prize is Shape Corp. from the United States. The prize is awarded for the groundbreaking use of martensitic steel in a 3D formed tube for automotive roof rail applications.
“With great skill, Shape Corp. has shown the way forward and has succeeded in efficiently utilizing a modern material with the highest possible strength level. This shows a major opportunity in challenging other lightweight…
More Releases for HyProtect™
Phase-out of 3M™ Novec™ Engineered Fluids
In December 2022, 3M™ announced their plans to stop producing per- and polyfluoroalkyl substances (PFAS) in its product line. Final order dates have now been set for March 31st 2025, and the supply of these products will end on December 31st 2025.
This phase-out affects the following 3M™ product lines:
3M™ Novec™ Engineered Fluids 3M™ Fluorinert™ Electronic Liquids
With 3M™ discontinuing these products, many companies are now evaluating alternatives to find suitable replacements.…
MetaFix™ PlantarMAX™ Foot Plate received FDA-Release
Merete Medical GmbH announces the FDA-approval for MetaFix™ PlantarMAX™ plate. PlantarMAX™ is designed as the first plantar/medial Lapidus fixation plate which takes advantage of the biomechanically superior fixation on the tension side of the Metatarsal/Cuneiform joint during a Lapidus Hallux Valgus correction procedure. The fixation on the tension side of the joint is superior to dorsal or medial fixation and offers additional compression as the patient proceeds to weight bearing.
Merete…
Dali Wireless™ Introduces its Low Power t-Series Solution: tHost™ and t30™
Palo Alto, October 25, 2012 – Dali Wireless, a leader in next generation wireless infrastructure, today announced the release of its low power t-Series solution, which consists of the t30 remote unit and the tHost head-end.
The exponential growth of mobile data capacity is challenging traditional cellular networks. Dali Wireless has created a unique solution to address these capacity limitations by combining industry standard protocols with the culmination of over…
GFI™ Software Announces GFI LANguard™ 2011
Clearwater, FL – May 23, 2011 – GFI Software’s Infrastructure Business Unit today announced the launch of GFI LANguard™2011, the latest version of the company’s comprehensive network vulnerability scanning and patch management solution. GFI LANguard 2011 is the first network vulnerability and patch management solution to integrate with more than 1,500 security applications and to include keyword search functionality, illustrating GFI’s commitment to providing small and medium-sized businesses (SMBs) with…
SaferWatch™ announces TIN Manager™
HEBRON, ND – SaferWatch announces the release of their latest software solution, TIN Manager™. The TIN Manager feature is designed to verify the accuracy of Taxpayer Identification Number (TIN) and name information prior to submitting 1099’s to the IRS so that the company will not be penalized or fined for mismatches.
TIN Manager eliminates tax preparation hassles by giving the bookkeeping departments of broker and shipper businesses a…
Powerboat Challenge™ now racing on the iPhone™
FISHLABS presents the multi-award-winning water racing spectacle with clearly improved graphics, analogue controls and rich realsound now also for the iPhone
Powerboat Challenge™ has achieved spectacular ratings on leading mobile games magazines like Pocketgamer, Airgamer and Mobilegame FAQS. For iPhone™ owners the racing game now offers an even better full-throttle experience with fast powerboats, cool dudes, hot babes, and cheeky comments. The new version is uncompromising in terms of sound and…
