Press release
Bio-Gate concludes another cooperation agreement with leading multinational implant manufacturer
Medical technology business further strengthenedCoating of orthopaedic revision implants with HyProtect technology
Focus on protection against infections with multi-resistant germs
Nuremberg/Bremen, 5 July 2022 - Bio-Gate AG (ISIN DE000BGAG981), a leading provider in the field of innovative health technologies, has concluded a contract with a leading multinational medical device manufacturer for the coating of orthopaedic implants in human medicine. As part of the cooperation and the joint project management, Bio-Gate is responsible for the application engineering and the production of the HyProtect coating and also supports the regulatory approval of the implants.
Infections with multi-resistant germs continue to be a major threat to people worldwide. The number of newly approved, more effective antibiotics has fallen by over 80 % since the 1980s, and at the same time the incidence of multi-resistant germs in hospitals has risen massively. Currently, the WHO speaks of 700,000 deaths annually in connection with antibiotic-resistant germs. If nothing is done in this context, the WHO predicts 10 million deaths and costs for the health system of 76 trillion euros for the year 2050.
Especially in the field of implant revisions, complications in the form of implant-associated infections and inflammations are associated with sometimes life-threatening consequences for the patient and extremely high costs for the healthcare system.
According to market researchers Frost & Sullivan, orthopaedics was the third largest sub-segment in medical technology worldwide in 2021. According to scientific publications (e.g. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States, Steven Kurtz et al.), the revision sector in the USA is expected to grow by at least 100 % by 2030, starting from 2022. This means about 260,000 knee revisions and 100,000 hip revisions in 2030. In comparison, primary care is expected to be 8.5 million knee revisions and 2.0 million hip revisions in 2030.
Numerous scientific publications have shown that Bio-Gate's HyProtect antimicrobial coating technology is able to kill multi-resistant germs. Since 2013, more than 110 people worldwide (Germany, Switzerland, Australia, New Zealand, UK, USA) have successfully received HyProtect-coated implants in the form of custom-implants and compassionate care cases (healing trials). So far, four scientific publications on individual cases have been published. Currently, scientists and surgeons are working on the collected publication of several cases from Germany as well as Australia and New Zealand.
The existing database on the HyProtect technology as well as the successful outcome of the single patient cases in particularly at-risk patients are key elements for the cooperation agreement with the multinational implant manufacturer. The customer, together with Bio-Gate, is in the process of preparing the regulatory approval documents. Good progress is already being made on the regulatory side. In addition to the remuneration for the project management and the coatings, Bio-Gate will also receive licence payments once the implants have been launched on the market.
Marc Lloret-Grau, CEO of Bio-Gate AG, says: "The recently concluded contract is another important step for us to successively expand the medical technology business field into another growth driver in the Bio-Gate Group. Now that we have already concluded the second important cooperation agreement with a multinational medical device provider, we also want to push ahead with talks with implant manufacturers in the USA."
Contact:
Bio-Gate AG
Neumeyerstr. 28-34
90411 Nuremberg
Germany
www.bio-gate.de
North America:
Karl Richter
RFH BioTek Inc.
Tel: 416-407-6120
rfh@rfhBioTek.com
Toronto, Canada
Gerd Rückel
rikutis consulting
gr@rikutis.de
presse@bio-gate.de
Tel. +49 (0) 911 477523-222
Mobil +49 (0)152 34221966
Disclaimer:
This publication constitutes neither an offer to sell nor an invitation to buy securities. The shares in Bio-Gate AG (the "Shares") may not be offered or sold in the United States or to or for the account or benefit of "U.S. persons" (as such term is defined in Regulation S under the U.S. Securities Act of 1933, as amended (the "Securities Act")). The securities have already been sold.
6th floor, Iso Roobertinkatu 20-22
About Bio-Gate AG
The medical technology company Bio-Gate AG develops and markets applications which use unique silver technology to help prevent infections and thus to improve health. Bio-Gate AG's specialty is using pure silver to treat materials and surfaces that are used in all areas of daily life - thus providing long-term and medically effective protection against bacteria, microorganisms and other pathogens. Bio-Gate AG works in three fields to supply a variety of products that provide antimicrobial protection: material enhancement, surface coating and testing the antimicrobial or anti-adhesive properties of materials. The Nuremberg-based company offers systems that stretch across the entire value-adding chain, from development to approval to production. For more information, please visit www.bio-gate.de.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Bio-Gate concludes another cooperation agreement with leading multinational implant manufacturer here
News-ID: 2676027 • Views: …
More Releases from INS Trade Media Service

Walki introduces a broad portfolio of recyclable materials for the growing froze …
Walki is answering to the growing demand for frozen food by expanding its portfolio of recyclable materials.
The global market for frozen food is expected to grow to 322 billion euros by 2026. Although the demand is growing globally across all age groups, it is especially popular among younger consumers.
"Frozen food is an ideal way to prolong shelf life without losing out on the vitamins. It is also an excellent way…
Dassiet and OrthoPets collaborate to provide safer and easier orthopedic care fo …
The teams combine Dassiet’s clinically proven medical and material innovations with OrthoPets' strong experience in custom pet orthoses to provide human level care for animals.
The material innovation company Dassiet, and the leading, US based pet orthosis manufacturer OrthoPets have started collaboration to create sustainable, safe and user-friendly pet healthcare products. Both companies want to first tackle the issues surrounding veterinary casting, splints, and bandages, and to provide vets, pets and…
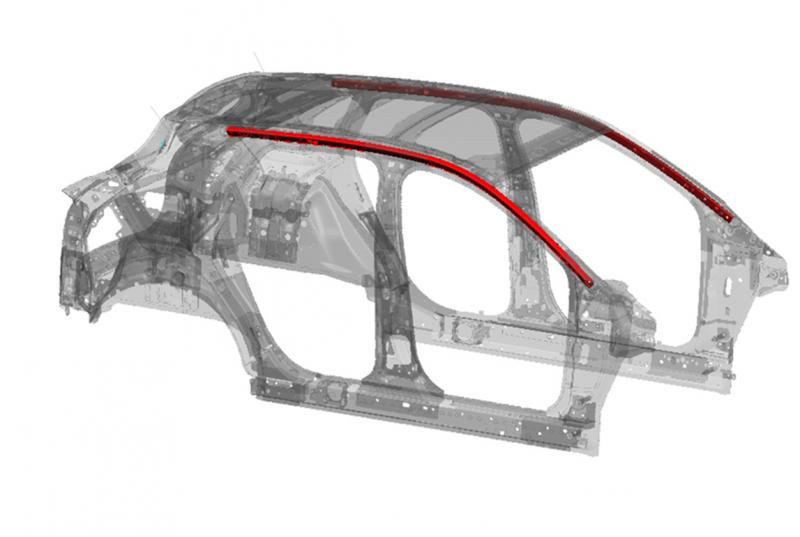
Shape Corp. wins Swedish Steel Prize 2019
The winner of the 20th international Swedish Steel Prize is Shape Corp. from the United States. The prize is awarded for the groundbreaking use of martensitic steel in a 3D formed tube for automotive roof rail applications.
“With great skill, Shape Corp. has shown the way forward and has succeeded in efficiently utilizing a modern material with the highest possible strength level. This shows a major opportunity in challenging other lightweight…

Extend your equipment’s performance and stay ahead of the competition with Alf …
Maintaining the edge over competitors is never easy. Operations must be continually improved, and production capabilities optimised. At Alfa Laval we understand these demands, and the requirement that your equipment performs at its best throughout its lifetime. We help keep you competitive by minimizing costs and maximising the return on your equipment investment. Our global service network and 360° Service Portfolio support your operations at all times, stopping problems before…
More Releases for HyProtect
Medical Device Coatings Market: Expected High CAGR with Key Players by 2024-2031 …
The Medical Device Coatings Market study by DataM Intelligence offer an in-depth analysis of the market, presenting insightful observations, statistics, historical data, and industry-validated market insights. The report delves into the competitive positioning of key companies, examining factors such as product offerings, pricing strategies, financial health, product portfolios, growth initiatives, and geographical reach.
Download a Free sample PDF (Use Corporate email ID to Get Higher Priority) at: - https://datamintelligence.com/download-sample/medical-device-coatings-market
What is the…
Global Orthopedic Veterinary Implants Market 2022 to 2031 Available in New Resea …
The Orthopedic Veterinary Implants Market ought to witness an explicit In Upcoming Years. With geographical barriers out of sight, track-and-trace programs are on the verge to reach their zenith in the timespan mentioned above. This could be credited to OEMs trying to reach out to the end-users remotely through virtual setups. Plus, people are getting a broader choice regarding choosing the experts. This holds for preventive as well as curative…
Orthopedic Veterinary Implants Market to grow based on digitized impertinence at …
The Orthopedic Veterinary Implants Market is slated to grow at a gracious rate of 8% by the year 2029. With value-oriented approach being the need of the hour, the healthcare vertical is likely to go the technologically advanced way in the next 10 years. With Big Data, AI comprising these advancements, the healthcare vertical is bound to create greater strides going forward.
A recent report by Grey2K USA suggests that more…
Orthopedic Veterinary Implants Market 2022 Trends, Share, Size, Growth, Opportun …
Orthopedic Veterinary Implants Market 2022
Orthopedic Veterinary Implants Market is bound to reach at a CAGR of 8% between 2019 - 2029. The current scenario is such that on-demand healthcare storage is being asked for. Cloud computing thus curtails operational expenses and capital as it simplifies sharing medical records, creates and maintains telehealth apps, and automates backend operations. This would be the scene with the healthcare vertical in the upcoming period.
Click…
AI-Backed Technologies to Give an Impetus to Orthopedic Veterinary Implants Mark …
The Orthopedic Veterinary Implants Market is expected to grow on a vigorous note shortly. Though telemedicine has turned out to be an easily accessible service, proper amalgamation with therapy decisions is of utmost importance. At-home lab tests are a good by-product of telehealth. Remote monitoring and diagnosis are cost-effective. It also saves on downtime. So, saving on time and investing in convenience would be one of the trends of the…
The Orthopedic Veterinary Implants Market to Witness Invigorating Trends
The Orthopedic Veterinary Implants Market is there to witness remarkable growth in the forecast period. The existing scenario implies the consumerization of healthcare. In other words, technology is relieving the hospital staff from providing an appropriate level of the care needed, that too, in a better way. Data-driven diagnosis has seen the light of the day in wake of habits and requirements of smartphone-oriented patients. This would be the future…
