Press release
MetaFix™ PlantarMAX™ Foot Plate received FDA-Release
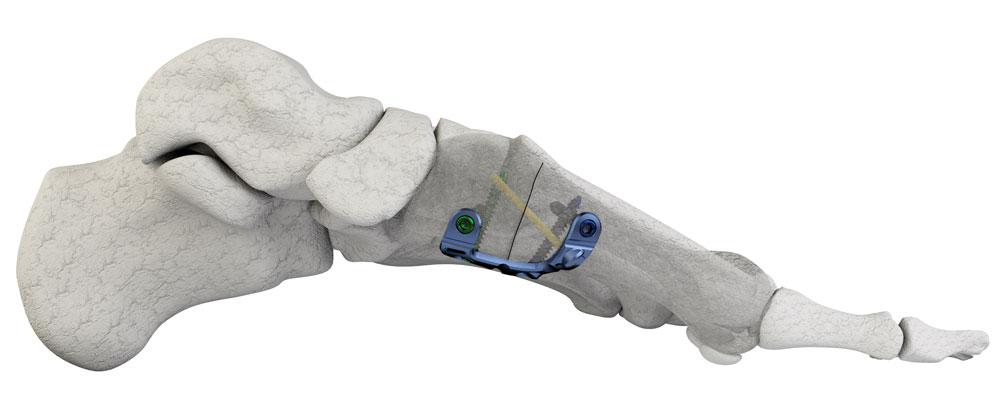
Merete Medical GmbH announces the FDA-approval for MetaFix™ PlantarMAX™ plate. PlantarMAX™ is designed as the first plantar/medial Lapidus fixation plate which takes advantage of the biomechanically superior fixation on the tension side of the Metatarsal/Cuneiform joint during a Lapidus Hallux Valgus correction procedure. The fixation on the tension side of the joint is superior to dorsal or medial fixation and offers additional compression as the patient proceeds to weight bearing.Merete Medical GmbH, founded in 1996, is an owner-operated medical technology company located in Berlin, Germany. The company focusses on innovative Research and Development and is producing individual solutions for hip revision, foot surgery, treatment of major bone defects, bone oncology, periprothetic fractures and trauma implants. The company sites are in Berlin (Germany), Luckenwalde (Germany), New York (USA) and Wroclaw (Poland).
Merete Medical, Inc.
Rudi Hilken
New York International Plaza
Stewart International Airport (SWF)
4 Crotty Lane – Suite 118
New Windsor, NY 12553
Tel. (914) 967-1532
Fax (914) 967-1542
E-Mail: rhilken@mereteusa.com
www.mereteusa.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release MetaFix™ PlantarMAX™ Foot Plate received FDA-Release here
News-ID: 262977 • Views: …
More Releases from Merete Medical GmbH

Merete Medical GmbH plans to expand its facilities in Luckenwalde and Berlin
July 4, 2014 - Merete Medical will expand its production capacity on the basis of the most modern manufacturing technologies in its manufacturing facilities in Luckenwalde Biotechnologie Park and at the Berlin site. These investments, spread over the next two years, aim to promote in particularly new and further developments in the successful and fast-growing segments arthroplasty / revision and foot reconstruction surgery. Overall, these investment measures aim at creating…