Press release
Embolic Protection Devices Market Forecast to 2024 Profiling Abbott, Cardinal Health, Medtronic & More
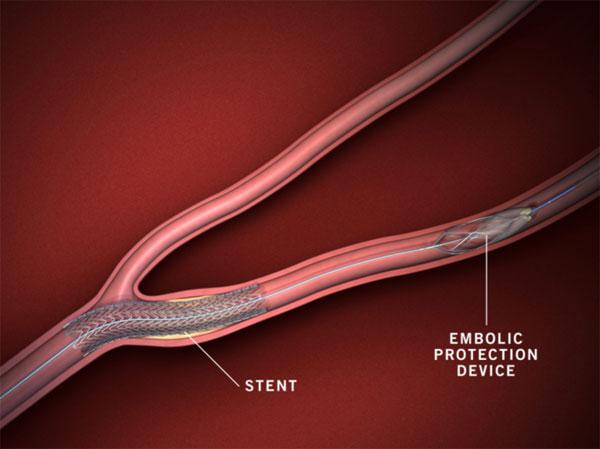
The latest Global Embolic Protection Devices industry forecast report covers the key technological advancement and market trends to 2024 and further lays out an analysis of the factors influencing the supply/demand for embolic protection devices with the opportunities/challenges faced by industry participants.The escalating occurrence of cardiovascular diseases, particularly among the geriatric population, thus, is a major driving force for Embolic Protection Devices Market. These components are prominently used in cardiac surgeries to reduce complications due to debris, owing to their superior design. Indeed, these devices also find extensive usage in ventricular assist device explantation and high-risk coronary artery bypass grafting. Driven by the nature of seriousness of these procedures, hospitals mandatorily prefer official FDA-approved EPD devices – a trend that is encouraging embolic protection devices market players to constantly improve their product portfolios.
Get a Sample Copy of this Report @ https://www.marketstudyreport.com/request-a-sample/934011/?utm_source=OPR-CC
An inherent vertical of the healthcare space, the competitive landscape of embolic protection devices market is defined by geographical expansions and M&As. Recently for instance, InspireMD, Inc., one of the global producers of embolic protection devices, signed an agreement with various regional distributors for extending its presence across myriad geographies.
In Greece, the firm partnered with T-Vascular IKE Medical Supplies and in the Caribbean, it has collaborated with SRL and Endo-Serv. With this exclusive distribution agreement, InspireMD is looking forward to attracting more customers for supplying highly advanced and minimally invasive medical devices. Another factor that may support the embolic protection devices industry player in its endeavor is that its regional distributors encompass a loyal, strong consumer base. Indeed, companies have been continually adopting such tactics to sustain in embolic protection devices market, the valuation of which stood at USD 870 million in 2016.
Recently in 2017, the U.S. based Claret Medical received clearance for its Sentinel Cerebral Protection System. With the development of this system, it has set a high standard of care in the United States to protect patient from strokes. Apparently, this system has the capability to remove the debris associated with TAVR (transcatheter aortic heart valve replacement).
As per statistics, the implementation of this novel system in TAVR has protected over 5,500 patients from the risk of stroke. In alignment with Claret, numerous other giants in embolic protection devices industry have been increasingly involved in the development of clinically advanced and next-generation minimally invasive medical devices. This would have considerable influence on product demand over the years ahead.
Apart from product differentiation, considering the future scope of highly advanced EPDs, companies in embolic protection devices industry have also been involved in the clinical trials of medical devices. The major concerns for these firms presently is safety and feasibility associated with the products. Recently, Contego Medical, LLC, a leading producer of medical devices for peripheral vascular and cardiovascular treatments, commenced a clinical trial for one of its drugs. The trial was conducted to evaluate the safety of the Neuroguard IEP® 3-in-1 Carotid Stent & Post-Dilation Balloon System with an Integrated Embolic Protection. With this development, Contego Medical has indeed added value to its neurovascular product portfolio and its research spectrum, encouraging other embolic protection devices industry biggies to do the same.
Prominent industry players include Abbott Laboratories, Cardinal Health, Medtronic, Boston Scientific, Gore Medical, Claret Medical, Allium Medical, Innovative Cardiovascular Solutions, Edward Lifesciences, and Transverse Medical among others. In order to further strengthen its competitive advantage, the market players are investing into research and development of embolic protection devices for expanded clinical applications such as cerebral emboli protection and lower extremities procedures.
Purchase this premium report at: https://www.marketstudyreport.com/securecheckout/paymenta/934011?msfpaycode=sumsf/?utm_source=OPR-CC
Numerous behemoths across embolic protection devices market have been aiming to set up novel benchmarks with regards to innovative product development. For instance, recently in 2017, Venus Medtech received the official approval from the China FDA for its Venus A-valve device. Apparently, this device offers a less invasive treatment solution for patients classified under the high-risk category. Medical device manufacturers thus, have been shifting their focus toward the development of highly advanced EPDs. Aided by their significant efforts, in conjunction with the emerging application arenas of these components, like atherectomy procedures, embolic protection devices industry is likely to garner appreciable gains over 2017-2024.
Related Report:
On a related note, another research has been added by Market Study Report in its database titled Reprocessed Medical Devices Market Size by Product, By End-use Industry Analysis Report, Regional, Application Potential, Competitive Market Share & Forecast, 2017 – 2024.
According to this research, U.S. held substantial revenue share owing to rising prevalence of heart disease, supportive regulatory framework, high number of medical products producers involved in the reprocessing programs. Moreover, encouraging governing policies along with established healthcare infrastructure upsurges reprocessed medical devices market growth during the forecast period. According to, Centers for Disease Control & Prevention (CDC) statistics updated in November 2017, nearly half of Americans have three risk factors of heart disease, these include high cholesterol, smoking, and high blood pressure.
Companies operational in the reprocessed medical devices includes Stryker Sustainability, Vanguard, GE Healthcare, Vascular Solutions, SureTek Medical, Centurion Medical Products, Soma Technology, Hygia Health Services, Steril Med (Ethicon Endo-Surgery), Mediline ReNewal, Midwest Reprocessing Centre, Medtronic, ReNu Medical, Pioneer Medical Devices, Steripro Canada, and Siemens Healthcare.
Read more on this report at: https://www.marketstudyreport.com/reports/reprocessed-medical-devices-market/?utm_source=OPRRR-cc
About Us:
Marketstudyreport.com allows you to manage and control all corporate research purchases to consolidate billing and vendor management. You can eliminate duplicate purchases and customize your content and license management.
Contact Us:
Market Study Report
The Green Suite #4594,
Dover, DE 19901
United States
Phone: 1-201-355-0868
US Toll Free: 1-866-764-2150
Email:sales@marketstudyreport.com
Website:https:/www.marketstudyreport.com
Blog: https://www.marketstudyreport.com/blog/
More Report at:http://reports.algosonline.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Embolic Protection Devices Market Forecast to 2024 Profiling Abbott, Cardinal Health, Medtronic & More here
News-ID: 994800 • Views: …
More Releases from Market Study Report

Wheelchair Tires Market Size to rise at 4.85% CAGR through 2028
Market Study Report recently added a new title on 2023-2028 Global Wheelchair Tires Market Report from its database. The report provides study with in-depth overview, describing about the Product, Size, Share, Industry Scope and elaborates market outlook and status to 2028.
Request Sample copy of this Report @ https://www.marketstudyreport.com/request-a-sample/5669807/
Global Wheelchair Tires Market Size was estimated at USD 402.34 million in 2022 and is projected to reach USD 560.42 million by 2028,…

Global Vital Signs Monitors Market Size to witness a CAGR of 0.1% during 2023-20 …
Market Study Report recently added a new title on 2023-2028 Global Vital Signs Monitors Market Report from its database. The report provides study with in-depth overview, describing about the Product, Size, Share, Industry Scope and elaborates market outlook and status to 2028.
Request Sample copy of this Report @ https://www.marketstudyreport.com/request-a-sample/5669877/
Global Vital Signs Monitors Market Size was estimated at USD 4811.69 million in 2022 and is projected to reach USD 4845.81 million…

Outboard Electric Motors Market Size to Record 6.26% CAGR Through 2028
Market Study Report: The Report 2023-2028 Global Outboard Electric Motors Market Report explores the essential factors of the Outboard Electric Motors market considering such as industry situations, market demands, market players adopted business strategies and their growth scenario. The Global Outboard Electric Motors market has been separated by this report based on the key players profiles, Type, Application and Regions.
Request Sample copy of this Report @ https://www.marketstudyreport.com/request-a-sample/5658245/ …

Robot Grippers Market Size growing at 13.33% CAGR to hit USD 5301.68 million by …
Global Robot Grippers Market Report added at Market Study Report offers industry size, share, growth, trends and forecast analysis up to 2028. Robot Grippers Market Report also covers top key players, porters five forces analysis and market segmentation in detail. This report examines the global Robot Grippers market and provides information regarding the revenue for the period 2023 to 2028.
Request Sample copy of this Report @ https://www.marketstudyreport.com/request-a-sample/5658423/ …
More Releases for Med
Elite Med Spa Partners with Salterra Marketing for Med Spa Growth in 2026
Elite Med Spa announces a 2026 partnership with Salterra Marketing to accelerate Med Spa Growth through local SEO, paid media, conversion optimization, and results tracking.
Elite Med Spa, a Scottsdale-based medical spa known for advanced aesthetics and body contouring, announced today a strategic partnership with Salterra Marketing to drive Med Spa Growth in 2026 through performance-focused digital strategy, local visibility, and conversion optimization.
The collaboration is designed to strengthen Elite Med Spa's…
10x Med Spa Marketing Launches to Help Med Spas Dominate Local Search, Paid Ads, …
Houston, TX - October 23, 2025 - 10x Med Spa Marketing [https://10xmedspamarketing.com/], a new performance-driven digital marketing agency founded by Martin Hristov, has officially launched to help med spas multiply their growth through data-driven SEO, website design, Google Ads, and paid social campaigns.
The agency's mission is to bridge the gap between modern med-spa marketing and measurable ROI - empowering clinic owners to attract high-value patients, increase bookings, and future-proof their…
Scottsdale Med Spa Unveils Comprehensive Injectable Treatment Services
Scottsdale Med Spa, a leading aesthetic treatment center located in the heart of Old Town Scottsdale, is proud to announce its comprehensive range of injectable treatments designed to help clients refresh, renew, and rejuvenate their appearance without surgery. Under the expert guidance of Dr. Vincent Marino, MD, and skilled injection specialist Melissa Newman, BSN, RN, CLT, the medical spa offers personalized care using the latest techniques in aesthetic medicine.
SCOTTSDALE, AZ…
Fetal Monitor Transducer market: New Prospects to Emerge by 2028 | Unimed Medica …
"
The Fetal Monitor Transducer global market is thoroughly researched in this report, noting important aspects like market competition, global and regional growth, market segmentation and market structure. The report author analysts have estimated the size of the global market in terms of value and volume using the latest research tools and techniques. The report also includes estimates for market share, revenue, production, consumption, gross profit margin, CAGR, and other key…
CAS MED SPA OFFERS COOLSCULPTING PROMOTION
MARIETTA, GEORGIA- APRIL 29, 2019- CAS MED SPA is excited to announce its CoolSculpting service, as well as a money saving promotional code. Available on their website at https://cs.cassmedspa.com/cas-coolsculpting-best-offer, patients can save $200 off their treatment. Going on through SPRING 2019, CAS MED SPA offers their best deal ever, up to $1500 with the purchase of six or more treatments!
CoolSculpting is a revolutionary way to eliminate body fat. Unlike liposuction…
EuropeSpa med quality standards published in book form
Internationally valid quality and safety criteria for health and medical wellness listed in a comprehensive compendium
Stuttgart/Wiesbaden, 3 September 2012 | The European Spas Association has published the EuropeSpa med criteria for medical spas and medical wellness providers. The book Quality Standard for Medical Spas and Medical Wellness Providers in Europe was unveiled today by publishing house Schweizerbart Verlag (www.schweizerbart.de) in Stuttgart.
For the first time, this compendium sets out about…