Press release
Cholesterol Lowering Biologic Drugs Market: Competitive Analysis Size and Major Regions Development Report
The cholesterol lowering biologic drugs offers a new, revolutionary alternative for people fighting with cholesterol with the same old treatments. The old method of treating cholesterol elevated levels includes a change in diet and consuming statin pills to prevent the body from making more bad cholesterol. The new class of PCSK-9 inhibitors is being approved by the FDA which works by helping the liver flush out the bad cholesterol instead of preventing the body from making low-density lipoproteins. The FDA has till now approved only two drugs Praluent and Repatha, both made from living things. The PCSK-9 is a protein which binds to the receptors, those which are responsible for getting the bad cholesterol out of the body.Request for Table of Contents @ https://www.persistencemarketresearch.com/toc/16048
The cholesterol lowering biologics drug market is expected to boom owing to the number of population with high cholesterol levels, sedentary lifestyle and increasing research developments. According to the Indian Council of Medical Research, at least three-fourth of the country’s population has dyslipidemia, a condition where the individual has abnormal levels of triglycerides or cholesterol. The risk factors are obesity, hypertension poor lifestyle habits and diabetes. Moreover, the demand for biologics is driven by the dramatic shift in production technology and an expansion of targeted diseases.
There are two types of cholesterol, the good and the bad cholesterol. High-density lipoproteins also known as good cholesterol is responsible for carrying the bad kind to the liver from where it is removed. However, the low density lipoproteins buildup in the arteries which may block them eventually. This decreases or blocks the blood flow from reaching the brain and the heart leading to cases of stroke or heart disease.
The demand for cholesterol lowering biologics drugs is expected to boom with the added advantages it offers over statins. For instance, both Praluent and Repatha are to be taken every two weeks. The FDA, for now, has approved these two biologics drugs for a specific type of hereditary high cholesterol, but the increasing research developments will expand its application for wider high cholesterol audience. In January 2017, the U.S. Federal District court has decided to remove Praluent from the market. The company has been given a 30 days times to appeal the injunction and work towards a solution.
In terms of geography, cholesterol lowering biologic drugs market has been divided into five regions including North- America, Europe, Asia- Pacific, Latin America and Middle-East & Africa. The population with high-level cholesterol and the presence of FDA-approved biologics is making North America region to dominate the cholesterol lowering biologic drugs market. CDC estimates more than 102 million American’s with high-level cholesterols compared to what’s considered healthy. Of that population of 102 million people, around 35 million have cholesterol levels high enough to put them at risk of heart disease.
Europe has already seen in few launches in biologics and will see more in coming years. The sales of biologics have overtaken the sales of traditional drugs as these therapies are targeted towards high-value therapies for the chronic condition.Asia-Pacific is the fastest growing region as the market of biosimilars across various players is growing at a high growth rate.
Request to View Sample of Research Report @ https://www.persistencemarketresearch.com/samples/16048
Few of the cholesterol lowering biologics drugs market participants are Amgen Inc., Regeneron Pharmaceuticals, Inc. and Alnylam Pharmaceuticals.
About Us
Persistence Market Research (PMR) is a third-platform research firm. Our research model is a unique collaboration of data analytics and market research methodology to help businesses achieve optimal performance.
To support companies in overcoming complex business challenges, we follow a multi-disciplinary approach. At PMR, we unite various data streams from multi-dimensional sources. By deploying real-time data collection, big data, and customer experience analytics, we deliver business intelligence for organizations of all sizes.
Contact Us
Persistence Market Research
305 Broadway
7th Floor, New York City,
NY 10007, United States,
Telephone - +1-646-568-7751
USA – Canada Toll Free: 800-961-0353
Email: sales@persistencemarketresearch.com
Web: http://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Cholesterol Lowering Biologic Drugs Market: Competitive Analysis Size and Major Regions Development Report here
News-ID: 944271 • Views: …
More Releases from Persistence Market Research

Cryogenic Storage Tanks Market Predicted to Hit US$ 12.8 Billion by 2033 Driven …
According to the latest study by Persistence Market Research, the global cryogenic storage tanks market is likely to be valued at US$ 8.6 billion in 2026 and is projected to reach US$ 12.8 billion by 2033, expanding at a CAGR of 5.8% during the forecast period 2026-2033. Rising demand for liquefied gases across energy, healthcare, food processing, and industrial manufacturing sectors is emerging as a key driver shaping the market's…
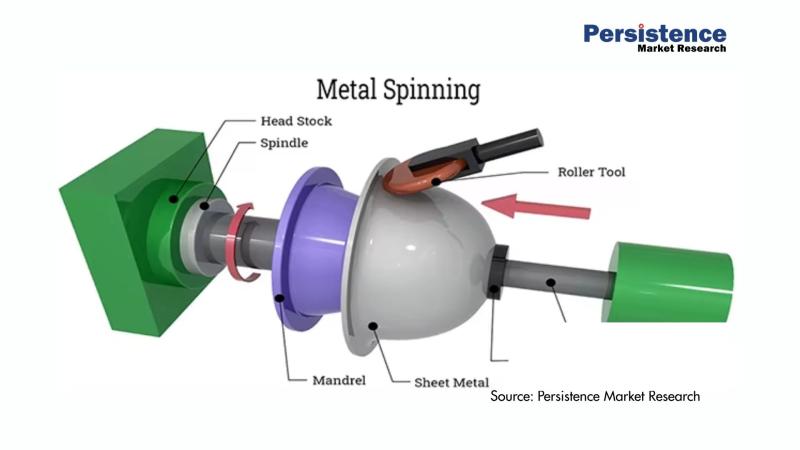
Metal Spinning Products Market Projected to Grow to US$ 4.0 billion by 2033 - Pe …
The global metal spinning products market is poised for substantial growth in the coming years. According to a recent study by Persistence Market Research, the market size is anticipated to reach US$ 4.0 billion by 2033, growing at a robust compound annual growth rate (CAGR) of 4.2% from its current valuation of US$ 3.0 billion in 2026. Metal spinning, a process of shaping metal into precise and symmetrical shapes, is…
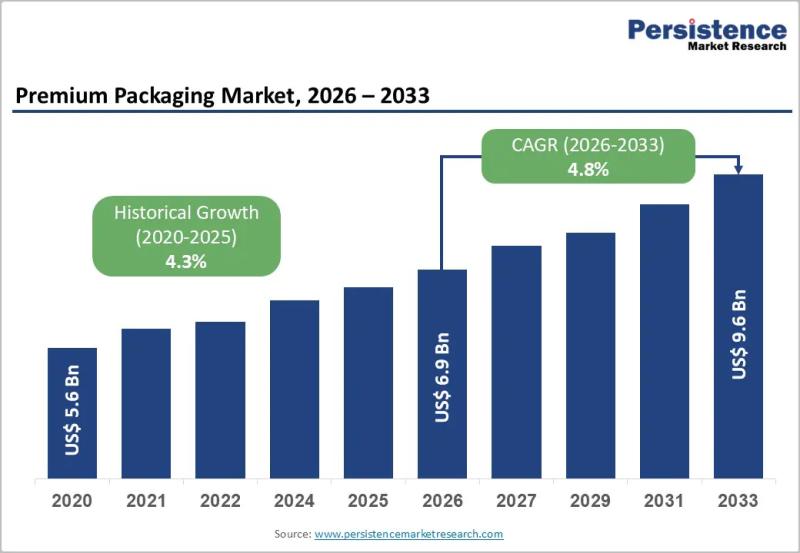
Premium Packaging Market Size Worth US$9.6 Billion by 2033 - Persistence Market …
The premium packaging market has evolved into a critical strategic element for brand differentiation across multiple high value consumer industries. Premium packaging goes beyond basic containment and protection to deliver enhanced aesthetics tactile appeal storytelling and emotional connection. Brands increasingly view packaging as an extension of their identity and a powerful marketing tool that influences purchasing decisions at the point of sale and during the unboxing experience. This shift is…
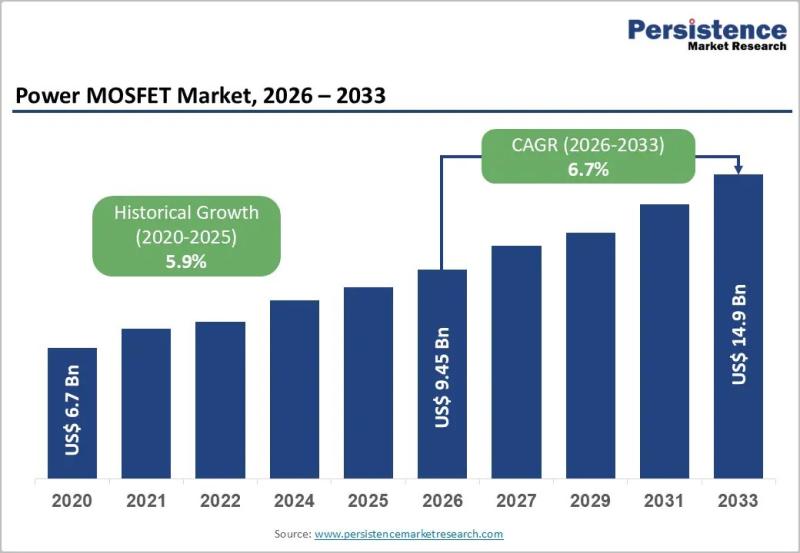
Power MOSFET Market Growth Driven by EVs Renewable Energy and Smart Automation
The global Power MOSFET market is entering a phase of sustained expansion, driven by the accelerating need for energy-efficient and high-performance power management components across industries. In 2026, the market is expected to be valued at US$ 9.45 billion and is forecast to reach US$ 14.9 billion by 2033, registering a healthy CAGR of 6.7% during the forecast period. Power MOSFETs are essential semiconductor devices that enable efficient switching and…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
