Press release
Trevigen Releases CultreCoat® Cell Adhesion Assays
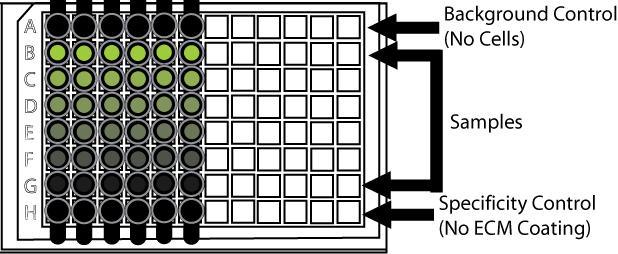
Trevigen announces the release of seven new coated well assay kits for cell adhesion studies.A cell’s ability to adhere to extracellular matrix (ECM) proteins is a necessary precursor to cell invasion, and this process also modulates morphogenesis, wound healing, proliferation, differentiation, and survival. Researchers studying cancer cell behavior or developing cancer therapeutics study cell adhesion with various proteins, recognizing that manipulation of cell adhesion to the extracellular environment may significantly impact cell activities. What is desired then is an assay system providing a variety of ECM proteins in a quantitative, high throughput format.
Trevigen answers this need with a simple, standardized, high throughput adhesion assay format offering 96 well plates coated with Basement Membrane Extract, Laminin I, Collagen I, Collagen IV, Fibronectin, Vitronectin, or an array of all six ECM proteins. A black stripwell format minimizes background providing increased sensitivity. Calcein labeling allows direct comparison between the number of cells loaded and the number of cells that adhere, and additional controls are provided for determining background and non-specific binding.
Highly qualified ECM proteins are also available separately from Trevigen.
Trevigen, Inc. focuses on the development, manufacturing and marketing of technology and products for cancer research, emphasizing apoptosis, DNA damage and repair, and cancer cell behavior.
Press Contact: Shamain Dang
Trevigen, Inc.
8405 Helgerman Court
Gaitherburg, MD 20877
www.trevigen.com
info@trevigen.com
Phone: 1-800-873-8443
Fax: 301-560-4973
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Trevigen Releases CultreCoat® Cell Adhesion Assays here
News-ID: 69494 • Views: …
More Releases from Trevigen, Inc.
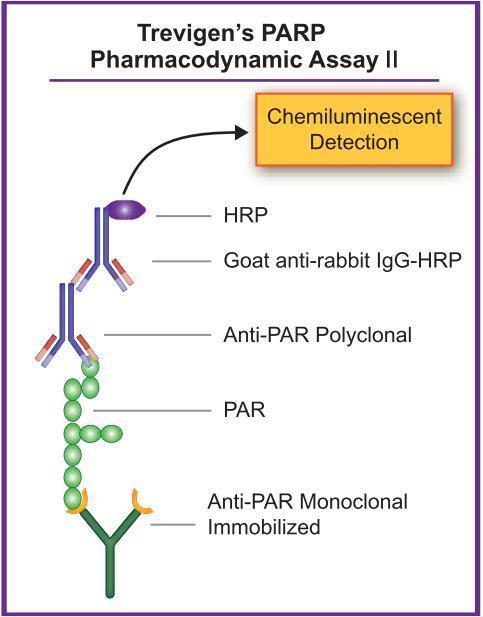
HT PARP in vivo Pharmacodynamic Assay II
Trevigen announces a validated assay, with higher sensitivity and pre-coated antibody plates to measure the effectiveness of PARP inhibitors in cell and tissue lysates for anticancer drug screening.
Pharmacodynamic (PD) assays have recently been developed and employed early in the drug screening process to assess the ability of potential drug candidates to affect molecular targets. These assays have the advantages of performing molecular proof-of-concept investigations at an early stage such as…

Trevigen Announces the First Standardized Comet Assay Electrophoresis System
The comet assay is the only direct method for the detection of DNA damage in cells. It is used in cancer research, in genotoxicity studies on environmental mutagens, and for screening compounds for cancer therapeutics. Based on single cell gel electrophoresis in a controlled pH environment, the assay allows the integrity of stained nuclear DNA to be examined and measured.
Until now, a lack of standardization in electrophoresis equipment, control cells,…
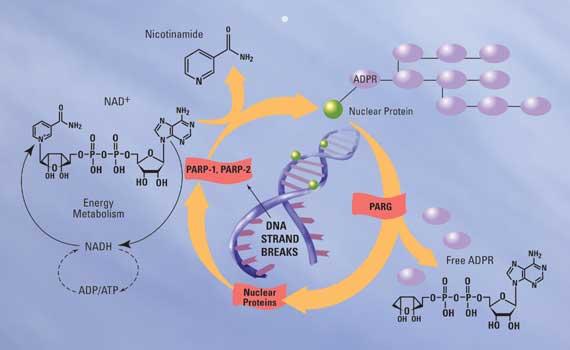
Trevigen Announces a Highly Sensitive, Fluorescent, One Hour PARP Inhibition Ass …
Poly(ADP-ribose) polymerase, through ribosylation of nuclear proteins, converts DNA Damage into intracellular signals that actuate either DNA repair or cell death. The development of inhibitors to PARP is of ever increasing interest in the drug discovery community since they can be used as modulators of DNA repair mediated resistance to cytotoxic cancer therapeutics. Until now, commercially available PARP assay kits required multiple steps and were time consuming.
Trevigen overcomes these…

Trevigen announces the release of Four New Assay Kits for 3-D Cell Proliferation
Many scientists are converting from growing cells in a traditional 2-D Monolayer on plastic surfaces with tissue culture medium to “3-D” Culture. In 3-D Culture, cells are grown in a microenvironment of specialized proteins which mimics in vivo conditions and allows the cells to organize into complex structures rather than uniform monolayers. Various types of cells require different specialized proteins for optimal growth conditions and these extracellular matrices can play…
More Releases for Assay
ProBio offers ADCC assay (SC1544) and CDC assay (SC1545) services for customers
ADCC & CDC assays
Endpoint-Based, and Speedy
Dramatic improvement of ADCC [https://www.probiocdmo.com/add-adcc-cdc-assay.html] by using recombinant NK cells
Antibody therapy has been proven to be highly powerful for cancer treatment. Two important mechanisms used by antibody drugs to kill targeted tumor cells are Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC), and Complement Dependent Cytotoxicity (CDC). ProBio is pleased to present to customers both a PBMC-based ADCC assay [https://www.probiocdmo.com/add-adcc-cdc-assay.html] and natural kill cell-based ADCC assay. The readout is…
Immunofluorescence Assay Market Report 2024 - Immunofluorescence Assay Market Si …
"The Business Research Company recently released a comprehensive report on the Global Immunofluorescence Assay Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive Sample…
ELISpot and FluoroSpot Assay Market Combating Chronic and Infectious Diseases: T …
ELISpot and FluoroSpot Assay Market worth $512.39 Mn by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ELISpot and FluoroSpot Assay Market- (By Product (Assay Kits (Assay Kits by Technique(ELISpot Assay Kits, FluroSpot Assay Kits), Assay Kits by Utility (Diagnostic Kits, Research Kits), Assay Kits by Analyte
(T-cell-based kits, B-cell-based kits, Other analytic kits), Analyzers,…
Creatinine Assay Kits Market
The global creatinine assay kits market is expected to reach USD 285.86 Million by 2025, from USD 192.07 Million in 2017 growing at a CAGR of 5.1% during the forecast period of 2018 to 2025. The upcoming market report contains data for historic years 2017, the base year of calculation is 2017 and the forecast period is 2018 to 2025.
Get Exclusive Sample Copy of This Report Here https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-creatinine-assay-kits-market
Some of the…
Lateral Flow Assay Market
Worldwide Lateral Flow Assay Market Analysis to 2027 is a specialized and in-depth study of the Lateral Flow Assay industry with a focus on the global market trend. The report aims to provide an overview of global Lateral Flow Assay market with detailed market segmentation by product & services /application and geography.
Get Sample PDF at https://www.theinsightpartners.com/sample/TIPRE00005544/?utm_source=OpenPR&utm_medium=Sumaiya
Lateral flow tests, also known as lateral flow immunochromatographic assays. The lateral flow assay…
ELISA Assay Dispensing Trends 2016
ReportsWorldwide has announced the addition of a new report ELISA Assay Dispensing Trends 2016 to its growing collection of premium market research reports.
This market report summarizes the results of HTStec's industry-wide global web-based benchmarking survey on enzyme-linked immunosorbent assays (ELISA) carried out in April 2016.
The survey was initiated by HTStec as part of its tracking of life science marketplaces and to update HTStec's previous ELISA Assay Trends report (June 2012).…