Press release
Breast Cancer Diagnostics Market Will hit at a CAGR of 4.7% by 2017 - 2022
Considering the prevalence of breast cancer, pharmaceutical companies are increasing their inputs on devising newer, more effective treatments for this baneful disease. At the same time, government initiatives are striving to control the occurrence rate by boosting awareness about breast cancer, its symptoms, and preventive measures.The report on global breast cancer diagnostics market analyzes the market’s expansion till date, and offers an in-depth forecast on how the market will shape up in the years to come. Stakeholders in the global breast cancer diagnostics market will gain actionable insights on the future direction of this market.
Influence of the Food & Drug Administration (FDA)
The FDA’s expanse is not limited to the US or North America; it monitors drug development and commercialization beyond borders. In the treatment of breast cancer, new therapies, surgical procedures and drugs are developed across multiple regions in the world. The FDA approval on such developments is instrumental in assessing the trajectory for the market’s growth in the coming years. While existing products have been successful in obtaining the nod, the FDA will not exhibit a lenient stance of manufacturers of such equipment.
Request For Report Sample: https://www.factmr.com/connectus/sample?flag=S&rep_id=55
Breast Cancer Diagnostics Market – Regional Outlook
The report includes a comprehensive segmentation of the global breast cancer diagnostics market by dividing the globe into regions such as North America, Europe, Latin America, Japan, Asia-Pacific excluding Japan (APEJ) and Middle East & Africa (MEA). In this section, the expansion of global breast cancer diagnostics market across these regions is tracked, observing active participation of native players and assessing the lasting impact of government & regulatory policies. Recent developments and research undertakings of breast cancer treatment societies across these regions are also traced, which are used for inferring a decisive scope of the market’s growth within a particular region.
Robust healthcare infrastructures in developed countries will encourage medical organizations in partnering with pharmaceutical companies for development of favorable reimbursement policies. Countries with high concentration of demographics susceptible to breast cancer will witness a surging demand for therapies and drugs on breast cancer. On the other hand, manufacturers of breast cancer medicine & therapeutic instruments will set up their manufacturing units in regions with ample resource availability, low wage costs and approbative production regulations.
Competitive Landscape
The report also provides a detailed study on the competitive landscape for breast cancer diagnostics market. Key players in the global breast cancer diagnostics market will be profiled in this section. Based on their contribution to the global market revenues, and their capabilities to boost the market’s expansion, the section will include a comparative study on the key players in the market. In the long run, established players in the global breast cancer diagnostics market are likely to focus on adoption of new technologies to improve their production capacities. With sufficient capital at their disposal, these companies can also foster research and development by commissioning research projects on drug developments.
Request For Discount: https://www.factmr.com/connectus/sample?flag=D&rep_id=55
New entrants in the global breast cancer diagnostics market are likely to stick to standard production procedures. Through scientific intervention, some of these companies are likely to improve the quality of their offerings by partnering with esteemed medical research organizations. Notable mergers and acquisitions are also tracked in this section, providing an improved understanding on dynamics of the global breast cancer diagnostics market, particularly from an investment standpoint.
Research Methodology
Fact.MR is committed to offer unbiased and independent market research solutions to its clients. Each market report of Fact.MR is compiled after months of exhaustive research. We bank on a mix of tried-and-tested and innovative research methodologies to offer the most comprehensive and accurate information. Our main sources of research include,
Primary research
Secondary research
Trade research
Focused interviews
Social media analysis
Browse Full Report: https://www.factmr.com/report/55/breast-cancer-diagnostics-market
ABOUT US:
Fact.MR is focused on offering transformative intelligence that inspires breakthroughs and innovation. We believe that the right decisions at the opportune time are integral to achieve extraordinary success. We are here to help you with your strategic decision making.
CONTACT:
Suite 9884
27 Upper Pembroke Street,
Dublin 2, Ireland
Phone: +353-1-6111-593
Email: sales@factmr.com
Website: https://www.factmr.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Breast Cancer Diagnostics Market Will hit at a CAGR of 4.7% by 2017 - 2022 here
News-ID: 653816 • Views: …
More Releases from Factmr
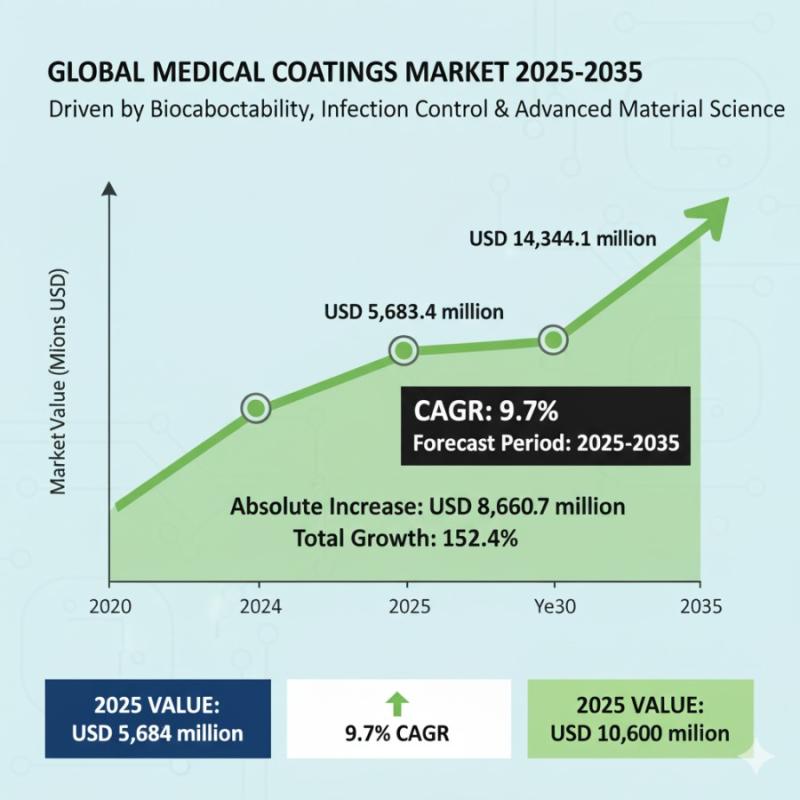
Medical Coatings Market to Hit USD 14,344.1 million by 2035- Growth Accelerates …
The global medical coatings market is set for sustained growth through 2035, powered by minimally invasive procedures, infection prevention priorities, and smart biocompatible innovations. According to Future Market Insights (FMI), the market is valued at USD 5,683.4 million in 2025 and is projected to reach USD 14,344.1 million by 2035, expanding at a compound annual growth rate (CAGR) of 9.7%.
The FMI report, "Medical Coatings Market Size, Share, and Forecast 2025-2035,"…
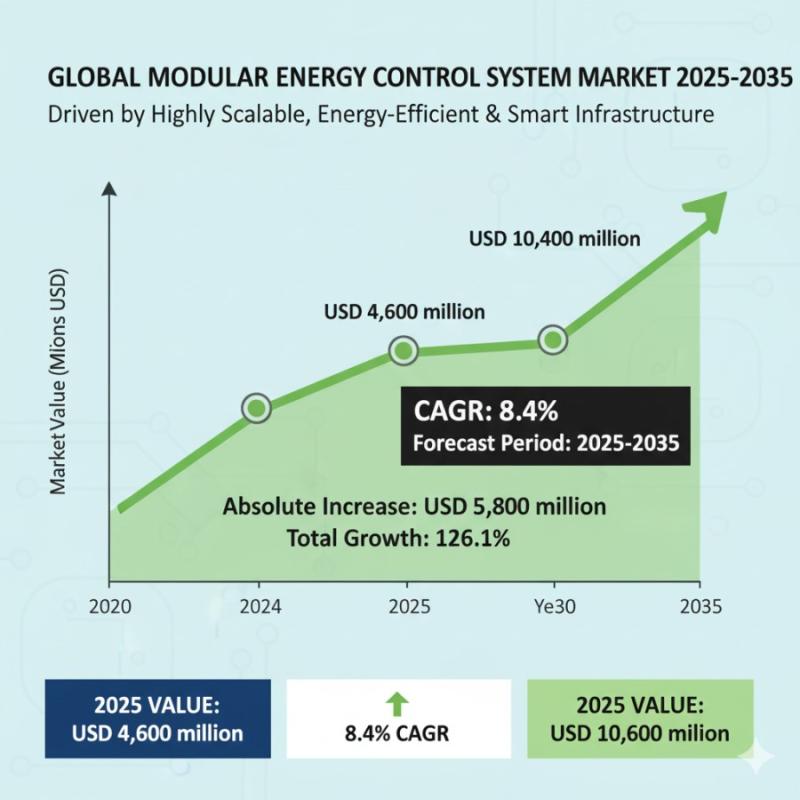
Modular Energy Control System Market to Hit USD 10,400 million by 2035- Growth A …
The global modular energy control system market is set for robust expansion through 2035, fueled by scalable infrastructure, real-time optimization, and seamless renewable energy integration. According to Future Market Insights (FMI), the market is valued at USD 4,600 million in 2025 and is projected to reach USD 10,400 million by 2035, expanding at a compound annual growth rate (CAGR) of 8.4%
The FMI report, "Modular Energy Control System Market Size, Share,…
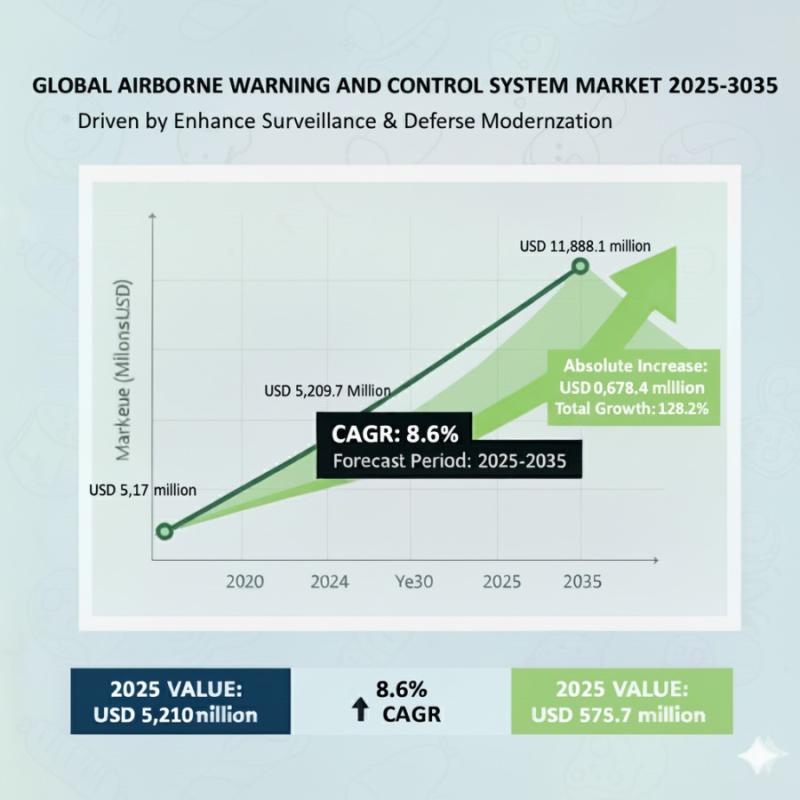
Airborne Warning and Control System Market to Surpass USD 11,888.1 million by 20 …
The global airborne warning and control system (AWACS) market is accelerating toward a decade of robust expansion, driven by escalating geopolitical tensions, defense modernization, and AI-enhanced threat detection. According to Future Market Insights (FMI), the market is valued at USD 5,209.7 million in 2025 and is projected to reach USD 11,888.1 million by 2035, growing at a compound annual growth rate (CAGR) of 8.6%.
The FMI report, "Airborne Warning and Control…
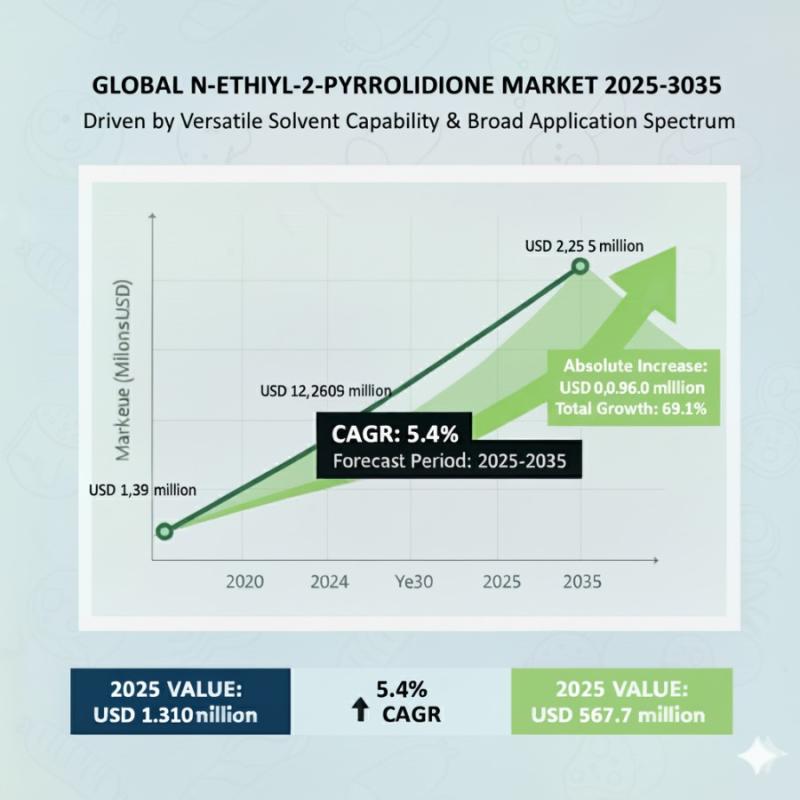
N-Ethyl-2-Pyrrolidone Market to Reach USD 2.35 million by 2035- Steady Growth Le …
The global N-Ethyl-2-Pyrrolidone (NEP) market is poised for consistent expansion through 2035, fueled by rising demand in high-purity electronics, lithium-ion battery production, and pharmaceutical synthesis. According to Future Market Insights (FMI), the market is valued at USD 1.39 million in 2025 and is projected to hit USD 2.35 million by 2035, growing at a compound annual growth rate (CAGR) of 5.4%.
The FMI report, "N-Ethyl-2-Pyrrolidone Market Size, Share, and Forecast 2025-2035,"…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
