Press release
Authorized Generics Market is Driven by Health Care Plans by Governments Across the World
A generic drug is a duplicate copy of original branded drug, which has same dosage form, active pharma ingredients (API), strength, route of administration, and also same intended use as the branded one. Regulatory authorities and governments have mandated that an authorized generic drug has to medicinally correspond to the branded drug and sanctioned as an Abbreviated New Drug Application (ANDA) by the Food and Drug Administration (FDA). An authorized generic is the branded company’s individual product, but repackaged and marketed as generic drug either via subsidiary or third party. These are already approved as a New Drug Application by the FDA, only they are promoted via private label.Browse Market Research Report @ http://www.transparencymarketresearch.com/authorized-generics-market.html
The authorized generics market grew rapidly in the past few years as these provide consumers with branded quality drugs at generic prices. Presently, there is a growing trend of original maker giving approvals to a subsidiary or a private label distribution company to sell its brand name drug as a generic drug at a subsequently low price.
An example of authorized generic drug is Azithromycin Pak which is sold under by the company name Greenstone. Pfizer’s original branded drug Z-pak was approved by the FDA. Before patent expiry of Z-pak, Pfizer allowed Greenstone to sell Z-pak using authorized generic name Azithromycin Pak. Greenstone is a wholly owned subsidiary of Pfizer.
The global authorized generics market is expected to witness strong growth. Authorized generic drugs are priced at significantly discounted rate i.e. 50% to 70% as compared to branded counterparts. Additionally, many of the popular branded drugs of pharma companies are losing patent protection rights, which is also termed as patent cliffs. This would pave the way for entry of new complex generics in the market. These factors are likely to drive the authorized generics market in the near future. Other factors driving the market are health care plans by governments across the world, rapidly increasing cost of branded drugs, and aging populations. On the other hand, possibility of side effects, and lack of regulatory awareness about products and quality management are factors likely to restrain the global authorized generics market.
The global authorized generics market can be classified based on product type, application, end-user, and region.
In terms of product type, the global authorized generics market can be segmented into biosimilars, simple generic, super generic, and others. Based on applications the market can be classified into cardiovascular, anti-infective, anti-arthritis, central nervous system, anti-cancer, respiratory, and others.
Geographically, the global authorized generics market can be divided into North America, Asia Pacific, Europe, Latin America, and Middle East & Africa. North America accounted for the largest share of the market, primarily due to technological advancements, rise in demand for generic drugs, increase in overall cost of branded drugs, and presence of key players. Europe held a significant share of the market attributed to advancements in generic drugs, rise in various types of cancer, and blood related disorders.
For more information on this report, fill the form @ http://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=20609
Asia Pacific, however, has been exhibiting high growth rate on account of growing demand for generic drugs, rise in geriatric population, increase in disposable income, and government initiatives to support generic drugs. Rapidly rising population in the region has increased demand for better health care and induced both private and government players to meet the demand. Developing economies such as India and China have also made a significant contribution to the rise of the global authorized generics market by focusing on establishing a better health care infrastructure.
Key players in the market are Teva Pharmaceuticals, Sandoz, Allergan, Mylan, Sun Pharmaceuticals, and STADA Arzneimittel. Other prominent vendors in the market are Abbott, Amgen, Apotex, Aspen, AstraZeneca, Aurobindo Pharma, Baxter, Berlin-Chemie, Biocon, Biogen, Boehringer Ingelheim, Celltrion, Cipla, Coherus Biosciences, Dr. Reddy's Laboratories, Daiichi Sankyo, Eli Lilly and Company, Emcure Pharmaceuticals, Eurofarma Laboratories, Gedeon Richter, Gilead Sciences, and GlaxoSmithKline.
About Us
Transparency Market Research (TMR) is a global market intelligence company providing business information reports and services. The company’s exclusive blend of quantitative forecasting and trend analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Contact Us
Transparency Market Research
State Tower,
90 State Street, Suite 700
Albany, NY 12207
United States
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: http://www.transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Authorized Generics Market is Driven by Health Care Plans by Governments Across the World here
News-ID: 648887 • Views: …
More Releases from Transparency Market Research

Metal Cans Market Valued at USD 71.7 Bn in 2025, Set to Reach USD 100.3 Bn by 20 …
The global Metal Cans Market was valued at US$ 71.7 Bn in 2025 and is projected to reach US$ 100.3 Bn by 2036, expanding at a steady CAGR of 3.1% from 2026 to 2036. The market's growth trajectory is supported by the accelerating transition toward sustainable and infinitely recyclable packaging formats, particularly in the beverage and food industries.
Explore pivotal insights and conclusions from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=706
North America…
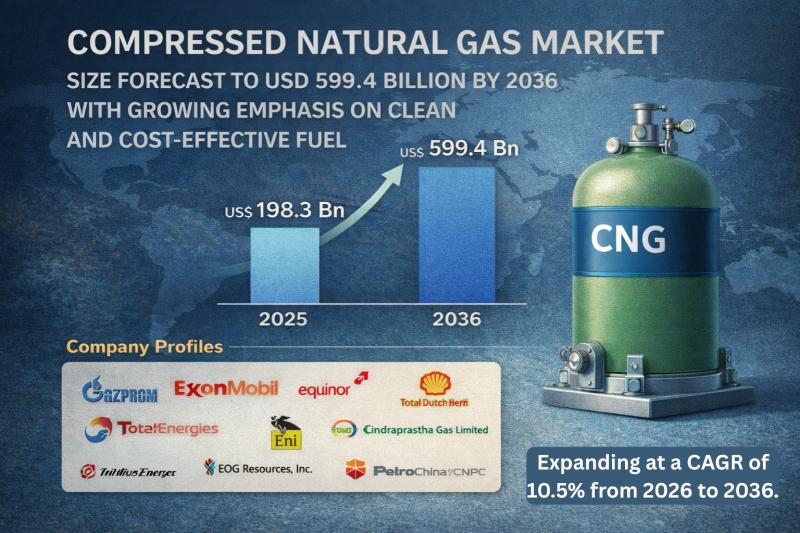
Compressed Natural Gas Market Size Forecast to USD 599.4 Billion by 2036 with Gr …
Compressed Natural Gas Market Outlook 2036
The global compressed natural gas (CNG) market was valued at US$ 198.3 Bn in 2025 and is projected to reach US$ 599.4 Bn by 2036, expanding at a robust CAGR of 10.5% from 2026 to 2036. Market growth is driven by rising demand for cleaner alternative fuels, supportive government policies promoting low-emission transportation, and expanding natural gas infrastructure worldwide.
👉 Get your sample market research report…
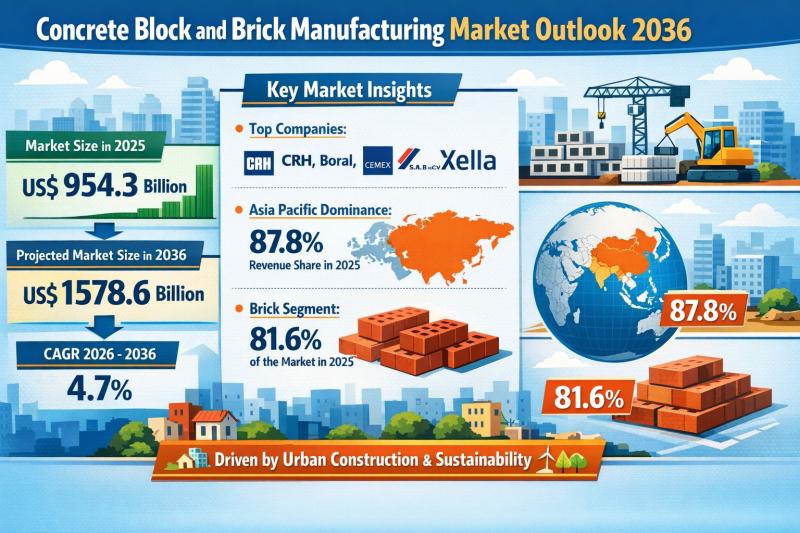
Concrete Block and Brick Manufacturing Market Outlook 2036: Surging Urban Infras …
The global concrete block and brick manufacturing market reached a valuation of US$ 954.3 billion in 2025 and is projected to expand to US$ 1,578.6 billion by 2036, growing at a compound annual growth rate (CAGR) of 4.7% from 2026 to 2036. The steady rise reflects the sustained demand for masonry materials across residential, commercial, and infrastructure projects worldwide.
Historical data from 2021 to 2025 demonstrates consistent volume growth, while forward…
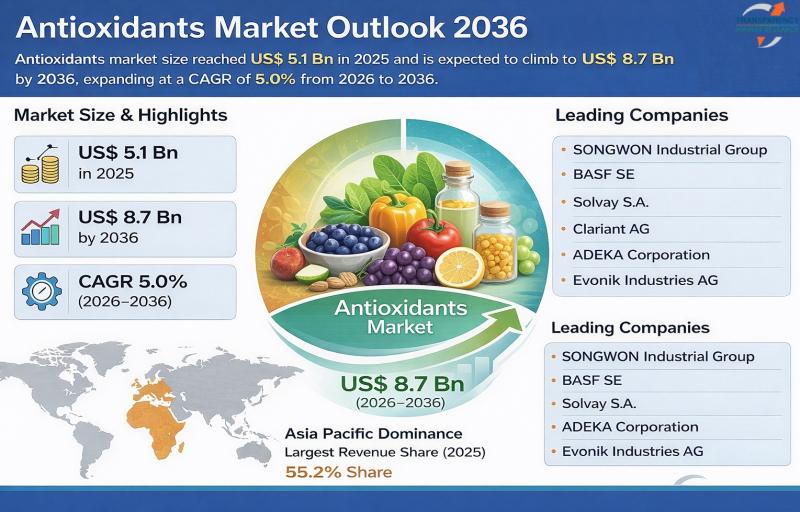
Antioxidants Market to Reach US$ 8.7 Billion by 2036, Fueled by Health Awareness …
The global antioxidants market was valued at US$ 5.1 Billion in 2025 and is projected to reach US$ 8.7 Billion by 2036, expanding at a CAGR of 5.0% from 2026 to 2036. The market is witnessing steady growth driven by increasing health awareness, expanding food preservation applications, rising demand for functional foods, and growing use of antioxidants in cosmetics and pharmaceuticals.
Antioxidants are compounds that inhibit oxidation and neutralize free radicals,…
More Releases for Authorized
Finsmart Accounting Becomes Zoho Authorized Partner
Leading accounting outsourcing company of India becomes Zoho partner to help businesses make the most of its comprehensive finance and accounting solutions.
Pune, India May 05, 2023 - Finsmart Accounting is known globally for offering offshore bookkeeping services in India. The company recently announced its partnership with Zoho to help organizations make the most of Zoho accounting and finance solutions.
Zoho's software and web tools are used by millions of…
CRM Masters Authorized Zoho Consulting Partner
Founded in 2016, CRM Masters Infotech is a Zoho Premium Partner and a Salesforce Certified Partner.CRM Masters is a trusted source for businesses to employ digital solutions with ultimate security. As Premium Zoho Consulting Partners, we offer exceptional and comprehensive implementation of the entire Zoho One suite - from CRM Plus and Books to People Plus & Creator- guaranteeing full GDPR & HIPPA compliance at all times! Unlock your business's…
Matrox adds ASI Canada as an Authorized Distributor
Partnership allows ASI to offer a wide range of graphics solutions to the Canadian market
MONTREAL — Nov. 7, 2013 — Fremont, California-based ASI Computer Technologies, a leading distributor of computer components and peripheral products to over 20,000 VARs throughout North America with branch locations in Toronto, Montreal, and Vancouver, has recently been added as a Matrox Graphics Authorized Distributor in Canada.
ASI has been an authorized distributor in the United States…
Palmsol Joins Google Apps Authorized Reseller Program
November 11, 2011-- Palmsol today announced it has become an authorized reseller of the Google Apps™ suite of communication and collaboration tools.
“The Google Apps Reseller program will help us enhance the value of Google Apps for businesses in South Florida," said Michael Zuloaga, managing partner of Palmsol – IT Solutions for Business. "Use of cloud computing, through solutions like Google Apps, is one way to innovate traditional…
Crowne Ventures To Reduce Number Of Authorized Shares
Gold mining and exploration growth stock company, Crowne Ventures, Inc. (Stock Symbol: CRWV), has announced that its Board of Directors and Stockholders have approved an Amendment to the Articles of Incorporation to reduce the number of authorized shares in the Company from 1,000,000,000 to 550,000,000, with 500,000,000 common shares authorized, and 50,000,000 preferred shares authorized.
In addition, the Company has announced the launch of its new website: http://www.CrowneMining.com.…
ASI Creates Authorized iMIS Fundraising Partner Program
ALEXANDRIA, Va., (June 8, 2011)—Advanced Solutions International (ASI), a leading global provider of web-based software for donor-based non-profits, today announced the company has accredited several solution providers and consultants for its global Authorized iMIS Fundraising Partner program based on the quality of their performance serving donor-based organizations. The organizations that have been accredited are existing authorized partners and were selected for this program as a result of their success and…
