Press release
Global Clinical Trial Management System Market is Expected to Surpass US$ 1,848.5 Million by 2019
According to a new market report published by Persistence Market Research “Global Market Study on Clinical Trial Management System: Asia to Witness Highest Growth by 2019” the global clinical trial management system market was valued at USD 844.0 million in 2013 and is expected to grow at a CAGR of 14% from 2014 to 2019, to reach an estimated value of USD 1,848.5 million in 2019.To get the view of full report @ http://www.persistencemarketresearch.com/mediarelease/clinical-trial-management-system-market.asp
Clinical trial is a medical research study performed on humans to check the safety and efficacy of drugs, devices and therapeutic products before they are finally launched in the market. Globally, the CTMS market is witnessing significant growth due to increasing R&D investment in pharmaceutical, life science and clinical research industries. It empowers organizations and research centers to enhance productivity and effectiveness of clinical trials by advancing and managing clinical trials. Integration of CTMS with hospital information system (HIS) provides more accurate and time saving documentation is also driving growth for the CTMS market. Additionally, increasing prevalence of diseases is supporting clinical trials in different regions, and increased clinical research outsourcing is playing a major role in the growth of the CTMS market. Increasing regulatory requirements in many countries has resulted in increased complexity for clinical trial protocols. Presence of various end users such as pharmaceuticals, clinical research organizations (CRO) and healthcare providers has increased the acceptance of CTMS. The global CTMS market was estimated to be USD 844 million in 2013. It is likely to grow at a CAGR of 14% during 2013 to 2019 to reach USD 1,848.5 million in 2019.
North America is a traditional clinical trial region. Due to regulatory and legal considerations and the clinical trial market has shifted from North America to developing countries such as India and China. Clinical trials in the U.S. have been funded and sponsored by National Institute of Health (NIH), other government agencies, academic groups, voluntary health organizations and industry.
In Europe, countries in Central and Eastern Europe provide abundant chance to life science companies for clinical development. Due to governmental support and funding for biomedical sciences, Germany has become a preferred location for clinical trials.
However, Asia is the fastest growing region in the clinical trial management system market.. Improved industry regulatory laws and patent expiration laws in various countries including Japan, China and India, have led to the expansion of the clinical trials market in Asia. Asia has lower cost of conducting clinical trials compared to Europe or the U.S.
A Sample of this report is available upon request @ www.persistencemarketresearch.com/samples/3017
Some of the major players in the CTMS market are Oracle Corporation, Bio-Optronics, MedNet Solutions, PAREXEL International Corporation, Medidata Solutions and BioClinica
To Buy Full Report for a Single User @ www.persistencemarketresearch.com/checkout/3017
About Us
Persistence Market Research (PMR) is a third-platform research firm. Our research model is a unique collaboration of data analytics and market research methodology to help businesses achieve optimal performance.
To support companies in overcoming complex business challenges, we follow a multi-disciplinary approach. At PMR, we unite various data streams from multi-dimensional sources. By deploying real-time data collection, big data, and customer experience analytics, we deliver business intelligence for organizations of all sizes.
Contact Us
Persistence Market Research
305 Broadway
7th Floor, New York City,
NY 10007, United States,
USA - Canada Toll Free: 800-961-0353
Email: sales@persistencemarketresearch.com
Web: http://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Clinical Trial Management System Market is Expected to Surpass US$ 1,848.5 Million by 2019 here
News-ID: 558443 • Views: …
More Releases from Persistence Market Research

Bicycle Spokes Market Set for Strong Growth at 5.4% CAGR Through 2032 - Persiste …
The global bicycle spokes market is rapidly gaining traction as bicycles continue to be adopted as preferred choices for commuting, fitness, recreation, and eco‐friendly mobility. The global bicycle spokes market size is likely to be valued at US$2.9 billion in 2025 and is expected to reach US$4.2 billion by 2032, registering a steady CAGR of 5.4 % between 2025 and 2032.
➤ Download Your Free Sample & Explore Key Insights: https://www.persistencemarketresearch.com/samples/30615
Bicycle…

Herbal Toothpaste Market Growth Poised at 6.5% CAGR Through 2033 Amid Rising Hea …
The global oral care industry is undergoing a transformational shift as consumers increasingly prioritize natural, chemical free alternatives. Central to this transformation is the herbal toothpaste market, which is rapidly emerging as a mainstream segment driven by rising health consciousness, sustainability trends, and demand for botanical formulations. The global herbal toothpaste market size is likely to be valued at US$ 2.6 billion in 2026 and is projected to reach US$…

Dead Sea Mud Cosmetics Market Set for Steady Expansion Amid Rising Demand for Na …
The global beauty and personal care industry continues to evolve as consumers shift toward natural, mineral-based, and wellness-oriented skincare solutions. Among these, Dead Sea mud cosmetics have gained strong traction for their mineral content and perceived therapeutic benefits. According to industry estimates, the global dead sea mud cosmetics market is likely to be valued at US$1.5 billion in 2026 and is projected to reach US$2.3 billion by 2033, expanding at…
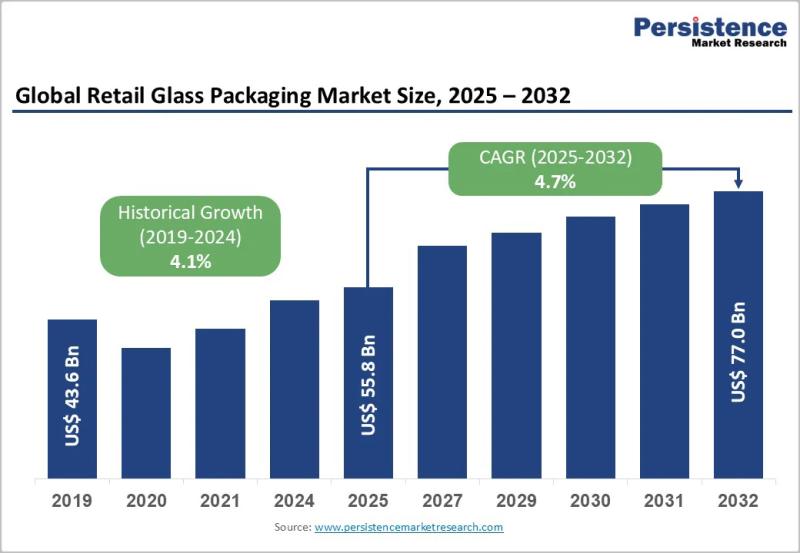
Retail Glass Packaging Market Projected to Reach US$77.0 Billion by 2032 at 5.3% …
The retail glass packaging market continues to play a crucial role in the global packaging ecosystem, particularly across food, beverage, cosmetics, and pharmaceutical retail channels. Glass packaging remains a preferred solution due to its premium appearance, chemical inertness, recyclability, and ability to preserve product integrity. As consumers increasingly prioritize sustainability, safety, and high quality packaging, retail glass packaging has regained strategic importance across both developed and emerging economies. Brands are…
More Releases for CTMS
Key Trends Shaping the Future Clinical Trial Management System CTMS Market From …
What Is the Estimated Market Size and Growth Rate for the Clinical Trial Management System CTMS Market?
The clinical trial management system (CTMS) market has experienced rapid growth in recent years. It is expected to grow from $1.41 billion in 2024 to $1.61 billion in 2025, with a CAGR of 14.2%. Growth factors include increasing clinical trial complexity, a rise in global clinical trials, regulatory compliance demands, an increasing focus on…
Clinical Trial Management System (CTMS) Market: Growth, Trends, Opportunities, a …
Introduction
A Clinical Trial Management System (CTMS) is an integrated software platform designed to streamline and manage the planning, execution, and monitoring of clinical trials. It helps healthcare organizations, pharmaceutical companies, research institutions, and contract research organizations (CROs) efficiently oversee the complex and data-driven processes of clinical trials. The CTMS enables management of trial activities such as patient recruitment, data collection, regulatory compliance, budgeting, and reporting.
Clinical trials are a critical component…
Clinical Trial Management System (CTMS) Market: Growth, Opportunities, and Chall …
Introduction
The Clinical Trial Management System (CTMS) market is a crucial segment of the global healthcare and pharmaceutical industries, which supports the efficient and accurate management of clinical trials. CTMS refers to software systems used by pharmaceutical, biotechnology, and contract research organizations (CROs) to streamline the planning, tracking, and management of clinical trials. These systems provide centralized data management for clinical trial activities, which helps improve the speed, quality, and compliance…
Clinical Trial Management System (CTMS) Global Market Report 2024 - Clinical Tri …
"The Business Research Company recently released a comprehensive report on the Global Clinical Trial Management System (CTMS) Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
According to The Business Research Company's, The…
Clinical Trial Management System (CTMS) Market Scope & Future Opportunities Till …
An exclusive Clinical Trial Management System (CTMS) Market research report has been fabricated through the in depth analysis of the market dynamics across five regions including North America, Europe, South America, Asia-Pacific, Middle East and Africa. The segmentation of the market by components, end users, and region was done based on the thorough market analysis and validation through extensive primary inputs from industry experts (key opinion leaders of companies, and…
Latest Clinical Trial (CTMS) Market 2022 | Detailed Report
ReportsnReports publishes the report titled Clinical Trial (CTMS) that presents a 360-degree overview of the market under one roof. The report is developed with the meticulous efforts of an enthusiastic and experienced team of experts, analyts, and researchers that makes the report a valuable asset for stakeholders to make robust decisions. This report also provides an in-depth overview of product type, specification, technology, and production analysis considering vital factors like…
